Plum pudding model

The plum pudding model is an early 20th century model of an atom (it was later found to be wrong). It was proposed by J.J. Thomson in 1904,[1] after the electron had been discovered by him, but before the atomic nucleus was discovered. Scientists knew that there was a positive charge in the atom that balanced out the negative charges of the electrons, making the atom neutral, but they did not know where the positive charge was coming from. Thomson's model showed an atom that has a positively charged medium, or space, with negatively charged electrons inside the medium. Soon after its proposal, the model was called a "plum pudding" model because the positive medium was like a pudding, with electrons like plums inside.
Development into modern atomic model
[change | change source]Rutherford's model
[change | change source]In 1909, not long after Thomson's model was proposed, Hans Geiger and Ernest Marsden made an experiment with thin sheets of gold, to test Thomson's model. Their professor, Ernest Rutherford, expected the results to prove Thomson correct, but their results were very different to what they were expecting. In 1911, Rutherford discovered that the positive charges come from tiny particles called protons, and that the protons were in a tiny center called the nucleus, and that the electrons were orbiting around the nucleus.
Niels Bohr's model
[change | change source]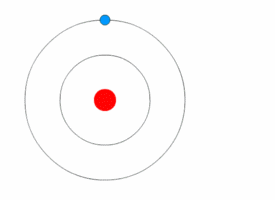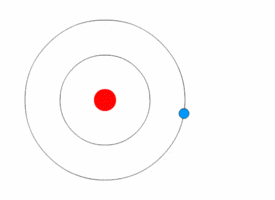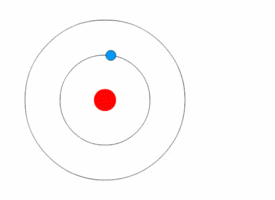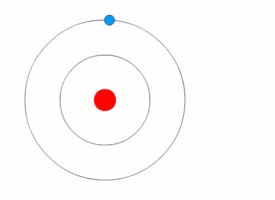
Rutherford's model was simple, but it was later disproved because electrons have charge, and they should be attracted to the positively charged nucleus. In 1913, Niels Bohr added 'energy levels' to the atomic model. Electrons do not fall into the nucleus because they are contained in energy levels, and to change to higher energy levels extra energy is needed, and to change to lower energy levels a release of energy is needed. It is not possible to change energy states without changing the energy of the electron. If an electron gets hit by a photon (a particle that carries electromagnetic radiation) it will gain extra energy and go into a higher energy level (it changes states), then it will jump back down to a lower energy level, releasing its contained energy. This new model was called the Bohr model or the Rutherford-Bohr model. This added a whole new branch of science: Quantum physics.
Quantum model
[change | change source]
In 1926 Erwin Schrödinger used the idea that electrons act as a wave, as well as a particle; this is known as a wave-particle duality. With a particle, one can know where it is in space when looking at it. But with a wave, it is all over the place, so there is no clear definition of where exactly it is. This is known as quantum uncertainty. With an electron, the probability of being in one place is known, because it is a wave as well as a particle.
Related pages
[change | change source]- Atomic theory
- Quantum mechanics
- John Dalton
- J. J. Thomson
- Ernest Rutherford
- James Chadwick
- Niels Bohr
- Arnold Sommerfeld
- Erwin Schrödinger
References
[change | change source]- ↑ "Plum Pudding Model". Universe Today. 27 August 2009. Retrieved 19 December 2015.