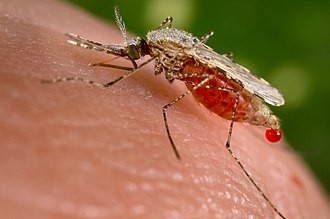
Anopheles stephensi is a primary mosquito vector of malaria in urban India and is included in the same subgenus as Anopheles gambiae, the primary malaria vector in Africa.[1] A. gambiae consists of a complex of morphologically identical species of mosquitoes, along with all other major malaria vectors; however, A. stephensi has not yet been included in any of these complexes.[2] Nevertheless, two races of A. stephensi exist based on differences in egg dimensions and the number of ridges on the eggs; A. s. stephensi sensu stricto, the type form, is a competent malaria vector that is found in urban areas, and A. s. mysorensis, the variety form, exists in rural areas and exhibits considerable zoophilic behaviour, making it a poor malaria vector.[3] However, A. s. mysorensis is a detrimental vector in Iran.[4] An intermediate form also exists in rural communities and peri-urban areas, though its vector status is unknown.[4] About 12% of malaria cases in India are due to A. stephensi.[5]
| Anopheles stephensi | |
|---|---|
| |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Diptera |
| Family: | Culicidae |
| Genus: | Anopheles |
| Subgenus: | Cellia |
| Species: | A. stephensi
|
| Binomial name | |
| Anopheles stephensi Liston, 1901
| |
In November 2015, an American research group demonstrated that an A. stephensi with genetic modifications could be rendered incapable of transmitting malaria, and that 99.5% of the mutant mosquitoes' offspring were also immune.[6]
In April of 2023, a malaria outbreak at Dire Dawa University in Ethiopia affected 1,300 students. The outbreak was a mystery because it occurred in the dry season and in an urban area, both atypical conditions for common cases of malaria in this area of the world. Blood tests confirming malaria's ring-shaped parasite ultimately led researchers to conclude that it was the work of A. stephensi, which thrives in urban areas and dry seasons, and has a resistance to insecticides.[7]
A team of scientists, headed by an entomologist from the University of Oxford, conducted an evaluation of Africa's environments to determine if they provide suitable conditions for the A. stephensi mosquito. Their findings indicate that the ongoing spread of this species could potentially expose an additional 126 million persons to the risk of malaria.[7]
Habitat
editIn rural areas, the larvae of A. stephensi may exist in many aquatic habitats, such as ponds, streams, swamps, marshes, and other sources of standing water.[8] They may also occupy smaller environments, such as tree holes, leaf axils, and man-made containers.[9] The larvae of A. s. mysorensis exclusively prefer to occupy stone pots and earthenware containers.[4] This species is also able to endure high levels of salinity, and have been found to breed readily in water where the salinity is equal to or even surpassing that of sea water.[10] Furthermore, A. stephensi breeds in a number of different water-bodies in urban areas, but predominantly in artificial containers, walls, overhead tanks, and ground level water tanks.[11]
Most larvae feed on microorganisms and particle matter suspended in water.[9] However, later in development, adult males feed on the nectar of flowers, whereas females take blood meals, which help produce viable eggs.[3]
Hosts
editHosts include Bos taurus, Mus musculus and Pimephales promelas.[12]
Parasites
editAnopheles stephensi is a vector of bovine leukemia virus[12] and Plasmodium berghei.[13] Mack and Vandenberg performed a series of experiments 1978-1979 finding that P. berghei derives nutrients from A. stephensi hemolymph during the sporogonic phase.[13] A. stephensi is an important vector for the human malaria species Plasmodium falciparum.[14]
Biochemistry
editMack and Vandenberg characterized A. stephensi's hemolymph composition in the late 1970s.[13]
Distribution
editAnopheles stephensi is a subtropical species that predominates in the Indian subcontinent (except Nepal and Sri Lanka)[3] and is also distributed across the Middle East and South Asia region, existing in countries such as: Afghanistan, Bahrain, Bangladesh, China, Egypt, India, Iran, Iraq, Oman, Pakistan, Saudi Arabia, and Thailand.[2] A. stephensi was discovered to be established on the continent of Africa, in Djibouti on the Horn of Africa in 2012[15] or 2013,[16] in 2016 in Ethiopia,[17][15] Sri Lanka in 2017,[15] and in 2019 in the Republic of the Sudan/North Sudan.[15]
Seasonal activity
editAnopheles stephensi is considered to be endophilic and endophagic, regardless that it may feed outdoors during the summer, when weather is warmer and humans and animals are more likely to sleep outside in the open air.[10] Although indoor feeding habits have shown no variation between seasons, adult females tend to feed more often at night during the summer rather than during the day in winter.[4] A. stephensi shows a greater preference for humans over animals in urban areas, where they can be found year-round.[4]
Insecticide resistance
editThe introduced African population appears to have arrived with several insecticide resistances.[15]
References
edit- ^ Valenzuela, Jesus G.; Francischetti, Ivo M.B.; Pham, Van My; Garfield, Mark K.; Ribeiro, José M.C. (2003). "Exploring the salivary gland transcriptome and proteome of the Anopheles stephensi mosquito". Insect Biochemistry and Molecular Biology. 33 (7). Elsevier: 717–732. doi:10.1016/s0965-1748(03)00067-5. ISSN 0965-1748. PMID 12826099.
- ^ a b Dash, A. P.; Adak, T.; Raghavendra, K.; Singh, O. P. (2007). "The biology and control of malaria vectors in India". Current Science. 92 (11). Current Science Association + Indian Academy of Sciences: 1571–1578. ISSN 0011-3891. JSTOR 24097721.
- ^ a b c Malhotra, Prithvi Raj; Jatav, Prakash Chandra; Chauhan, Ram Singh (2000). "Surface morphology of the egg of anopheles stephensi stephensi sensu stricto (diptera, culicidae)". Italian Journal of Zoology. 67 (2). Taylor & Francis: 147–151. doi:10.1080/11250000009356307. ISSN 1125-0003.
- ^ a b c d e Sinka, M.E., Bangs, M.J., Manguin, S., Chareonviriyaphap, T., Patil, A.P., Temperley, W.H., Gething, P. W., Elyazar, I.R.F., Kabaria, C.W., Harbach, R.E., & Hay, S.I. (2011). The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasites & Vectors, 4, 1-46.
- ^ Tikar, S. N.; Mendki, M.J.; Sharma, A. K.; Sukumaran, D.; Veer, Vijay; Prakash, Shri; Parashar, B. D. (2011). "Resistance Status of the Malaria Vector Mosquitoes, Anopheles stephensi and Anopheles subpictus Towards Adulticides and Larvicides in Arid and Semi-Arid Areas of India". Journal of Insect Science. 11 (85). Entomological Society of America (OUP): 1–10. doi:10.1673/031.011.8501. ISSN 1536-2442. PMC 3281435. PMID 21870971.
- ^ Gemuteerde mug moet malaria bestrijden Nederlandse Omroep Stichting 24 November 2015 (in Dutch)
- ^ a b Nolen, Stephanie; Negeri, Tiksa (29 September 2023). "An Invasive Mosquito Threatens Catastrophe in Africa". The New York Times. Retrieved 18 February 2024.
- ^ Rueda, Leopoldo M. (2007-12-18). "Global diversity of mosquitoes (Insecta: Diptera: Culicidae) in freshwater". Hydrobiologia. 595 (1). Springer: 477–487. doi:10.1007/s10750-007-9037-x. ISSN 0018-8158. S2CID 43415941.
- ^ a b Harbach, Ralph E. (2007-12-21). "The Culicidae (Diptera): a review of taxonomy, classification and phylogeny". Zootaxa. 1668 (1). Magnolia Press: 591–638. doi:10.11646/zootaxa.1668.1.28. ISSN 1175-5334. S2CID 17311770.
- ^ a b Manouchehri, AV; Javadian, E; Eshighy, N; Motabar, M (1976). "Ecology of Anopheles stephensi Liston in southern Iran". Tropical and Geographical Medicine. 28 (3): 228–32. CiteSeerX 10.1.1.455.2712. ISSN 0041-3232. PMID 1006792.
- ^ Jeyabalan, D; Arul, N; Thangamathi, P (2003). "Studies on effects of Pelargonium citrosa leaf extracts on malarial vector, Anopheles stephensi Liston". Bioresource Technology. 89 (2). Elsevier: 185–189. doi:10.1016/s0960-8524(03)00036-1. ISSN 0960-8524. PMID 12699939.
- ^ a b "Anopheles stephensi". Invasive Species Compendium (ISC). CABI (Centre for Agriculture and Bioscience International). 2019-11-22. Retrieved 2022-01-22.
- ^ a b c Vanderberg, Jerome P. (2009). "Reflections". Vaccine. 27 (1). Edward Jenner Society + Japanese Society for Vaccinology (Elsevier): 2–9. doi:10.1016/j.vaccine.2008.10.028. ISSN 0264-410X. PMC 2637529. PMID 18973784.
- ^ "Mosquitos, mosquito bite, mosquito transmitted diseases".
- ^ a b c d e "Vector alert: Anopheles stephensi invasion and spread". WHO. 2019-08-26. Retrieved 2020-11-11.
- ^ Faulde, Michael K.; Rueda, Leopoldo M.; Khaireh, Bouh A. (2014). "First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa". Acta Tropica. 139. Elsevier BV: 39–43. doi:10.1016/j.actatropica.2014.06.016. ISSN 0001-706X. PMID 25004439.
- ^ Carter, Tamar E.; Yared, Solomon; Gebresilassie, Araya; Bonnell, Victoria; Damodaran, Lambodhar; Lopez, Karen; Ibrahim, Mohammed; Mohammed, Seid; Janies, Daniel (December 2018). "First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches". Acta Tropica. 188: 180–186. doi:10.1016/j.actatropica.2018.09.001. ISSN 0001-706X. PMID 30189199.
Further reading
edit- "Vector alert: Anopheles stephensi invasion and spread". WHO. 2019-08-26. Retrieved 2020-11-11.
- Takken, Willlem; Lindsay, Steve (2019). "Increased Threat of Urban Malaria from Anopheles stephensi Mosquitoes, Africa". Emerging Infectious Diseases. 25 (7). Centers for Disease Control and Prevention (CDC): 1431–1433. doi:10.3201/eid2507.190301. ISSN 1080-6040. PMC 6590739. PMID 31063455.