Radiation/Electromagnetics

Electromagnetics are most familiar as light, or electromagnetic radiation. They span a spectrum from gamma rays to radio waves.
Gamma rays
[edit | edit source]
Def. very high frequency (and therefore very high energy) electromagnetic radiation emitted as a consequence of radioactivity is called a gamma ray.
Def. electromagnetic radiation consisting of gamma rays is called gamma radiation.
Gamma rays typically have frequencies above 10 exahertz (or >1019 Hz), and therefore have energies above 100 keV and wavelengths less than 10 picometers (less than the diameter of an atom]. However, this is not a hard and fast definition, but rather only a rule-of-thumb description for natural processes. Gamma rays from radioactive decay are defined as gamma rays no matter what their energy, so that there is no lower limit to gamma energy derived from radioactive decay. Gamma decay commonly produces energies of a few hundred keV, and almost always less than 10 MeV. In astronomy, gamma rays are defined by their energy, and no production process need be specified. The energies of gamma rays from astronomical sources range over 10 TeV, at a level far too large to result from radioactive decay. A notable example is extremely powerful bursts of high-energy radiation normally referred to as long duration gamma-ray bursts, which produce gamma rays by a mechanism not compatible with radioactive decay.
"The unusually wide span of the gamma-ray spectral window [covers] at least ten decades of photon energies (~105 - 1015 eV)".[1]
Hard gamma rays
[edit | edit source]"At higher energies [above 10 GeV], gamma rays are rare."[2]
"U-232 decays into Tl-208, a HARD gamma emitter. Thallium-208 emits hard 2.6 MeV gamma-rays as part of its nuclear decay. These gamma rays destroy the electonics and explosives that control detonation. They require thick lead shielding and have a distinctive and easily detectable signature. [...] Uranium-232 follows the same decay chain as thorium-232, but it follows it millions of times faster! This is because 232Th has a 14 billion-year half-life, but 232U has only an 74 year half-life! Once it starts down the hill it gets to thallium-208 (the gamma emitter) in just a few weeks! [...] Some 232U starts decaying immediately."[3]
Soft gamma rays
[edit | edit source]Soft gamma rays range from 120 keV to about 10 GeV.
X-rays
[edit | edit source]Def. an action or process of throwing or sending out a traveling X-ray in a line, beam, or stream of small cross section is called X-radiation.
X-rays span 3 decades in wavelength, frequency and energy.
Although the more energetic X-rays, photons with an energy greater than 30 keV (4,800 aJ) can penetrate the air at least for distances of a few meters (they would never have been detected and medical X-ray machines would not work if this was not the case) the Earth's atmosphere is thick enough that virtually none are able to penetrate from outer space all the way to the Earth's surface. X-rays in the 0.5 to 5 keV (80 to 800 aJ) range, where most celestial sources give off the bulk of their energy, can be stopped by a few sheets of paper; ninety percent of the photons in a beam of 3 keV (480 aJ) X-rays are absorbed by traveling through just 10 cm of air.
Hard X-rays
[edit | edit source]From 0.1 nm to 0.01 nm (about 12 to 120 keV) are hard X-rays.
Soft X-rays
[edit | edit source]From 10 to 0.1 nanometers (nm) (about 0.12 to 12 keV) they are classified as soft x-rays.
Super soft X-rays
[edit | edit source]Super soft X-ray sources (SSXSs) are in most cases only detected below 0.5 keV.
Ultrasoft X-rays
[edit | edit source]Ultra-soft x-rays are also known as grenz-rays (GRs).[4]
"The treatment was given twice a week over 3 consecutive weeks in total doses of 100 to 160 Gy. Dosage depended on the stage of [Lentigo maligna] LM and the depth of periadnexal atypical melanocytic extension in histologically examined materials before treatment."[4]
Ultraviolets
[edit | edit source]
Def. "the spectral region bounded on the long wavelength side at about λ3000 by the onset of atmospheric ozone absorption and on the short wavelength side at λ912 by the photoionization of interstellar hydrogen" is called the ultraviolet.[5]
The draft ISO standard on determining solar irradiances (ISO-DIS-21348)5 describes the following ranges:
| Name | Abbreviation | Wavelength range in nanometers | Energy per photon |
|---|---|---|---|
| Before UV spectrum | Visible light | above 400 nm | below 3.10 eV |
| Ultraviolet A, long wave, or black light | UVA | 400 nm–315 nm | 3.10–3.94 eV |
| Near | NUV | 400 nm–300 nm | 3.10–4.13 eV |
| Ultraviolet B or medium wave | UVB | 315 nm–280 nm | 3.94–4.43 eV |
| Middle | MUV | 300 nm–200 nm | 4.13–6.20 eV |
| Ultraviolet C, short wave, or germicidal | UVC | 280 nm–100 nm | 4.43–12.4 eV |
| Far | FUV | 200 nm–122 nm | 6.20–10.2 eV |
| Vacuum | VUV | 200 nm–100 nm | 6.20–12.4 eV |
| Low | LUV | 100 nm–88 nm | 12.4–14.1 eV |
| Super | SUV | 150 nm–10 nm | 8.28–124 eV |
| Extreme | EUV | 121 nm–10 nm | 10.2–124 eV |
| Beyond UV range | X-rays | below 10 nm | above 124 eV |
"Vacuum UV" is so named because it is absorbed strongly by air and is, therefore, used in a vacuum. In the long-wave limit of this region, roughly 150–200 nm, the principal absorber is the oxygen in air.
Middle ultraviolets
[edit | edit source]
As shown in the image on the right calcite fluoresces blue under short wave ultraviolet light.
Near ultraviolets
[edit | edit source]
As shown in the image on the right calcite fluoresces pink under long wave ultraviolet light.
Opticals
[edit | edit source]
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it.[6] Optics usually describes the behavior of visible, ultraviolet, and infrared light. Geometric optics treats light as a collection of rays that travel in straight lines and bend when they pass through or reflect from surfaces. Physical optics is a more comprehensive model of light, which includes wave effects such as diffraction and interference that cannot be accounted for in geometric optics.
Although visible light itself extends from approximately 4000 Å to 7000 Å (400 nm to 700 nm),[7] the same equipment used at these wavelengths is also used to observe some near-ultraviolet and near-infrared radiation.
Calcite has the chemical formula CaCO3.[8]
Calcite contains one oxide: CO3, or carbonate. It is 50 molecular % carbonate and 50 at % calcium.
Polars
[edit | edit source]

Light given off by a star is un-polarized, i.e. the direction of oscillation of the light wave is random. However, when the light is reflected off the atmosphere of a planet, the light waves interact with the molecules in the atmosphere and they are polarized.[9]
On the right is a photomicrograph of olivine in cross-section under crossed polarizers from an Archaean Kormatite in Australia.
Visuals
[edit | edit source]
| Color | Frequency | Wavelength |
|---|---|---|
| violet | 668–789 THz | 380–450 nm |
| blue | 631–668 THz | 450–475 nm |
| cyan | 606–630 THz | 476–495 nm |
| green | 526–606 THz | 495–570 nm |
| yellow | 508–526 THz | 570–590 nm |
| orange | 484–508 THz | 590–620 nm |
| red | 400–484 THz | 620–750 nm |
The visible spectrum is the portion of the electromagnetic spectrum that is visible to (can be detected by) the human eye. Electromagnetic radiation in this range of wavelengths is called visible light or simply light. A typical human eye will respond to wavelengths from about 390 to 750 nm.[10] In terms of frequency, this corresponds to a band in the vicinity of 400–790 THz. A light-adapted eye generally has its maximum sensitivity at around 555 nm (540 THz), in the green region of the optical spectrum (see: luminosity function).
There are several cases of astronomers who claimed that following a cataract operation, they could see shorter wavelengths than other people, slightly into the ultraviolet.
On the right, is an example of almost completely clear fluorite (CaF2).
Impurities can give color. Some halogen minerals are called halides.
Fluorescences
[edit | edit source]
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. However, when the absorbed electromagnetic radiation is intense, it is possible for one electron to absorb two photons; this two-photon absorption can lead to emission of radiation having a shorter wavelength than the absorbed radiation. The emitted radiation may also be of the same wavelength as the absorbed radiation, termed "resonance fluorescence".[11]
The most striking examples of fluorescence occur when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, and the emitted light is in the visible region.
"The common fluorescent lamp relies on fluorescence. Inside the glass tube is a partial vacuum and a small amount of mercury. An electric discharge in the tube causes the mercury atoms to emit ultraviolet light. The tube is lined with a coating of a fluorescent material, called the phosphor, which absorbs the ultraviolet and re-emits visible light. Fluorescent lighting is more energy-efficient than incandescent lighting elements. However, the uneven spectrum of traditional fluorescent lamps may cause certain colors to appear different than when illuminated by incandescent light or daylight. The mercury vapor emission spectrum is dominated by a short-wave UV line at 254 nm (which provides most of the energy to the phosphors), accompanied by visible light emission at 436 nm (blue), 546 nm (green) and 579 nm (yellow-orange). These three lines can be observed superimposed on the white continuum using a hand spectroscope, for light emitted by the usual white fluorescent tubes. These same visible lines, accompanied by the emission lines of trivalent europium and trivalent terbium, and further accompanied by the emission continuum of divalent europium in the blue region, comprise the more discontinuous light emission of the modern trichromatic phosphor systems used in many compact fluorescent lamp and traditional lamps where better color rendition is a goal.[12]
Ultraviolet lamps are also used in analyzing minerals and gems. Materials may look the same under visible light, but fluoresce to different degrees under ultraviolet light, or may fluoresce differently under short wave ultraviolet versus long wave ultraviolet.
Ultraviolet lamps may cause certain minerals to fluoresce, and is a key tool in prospecting for tungsten mineralisation.
Luminescences
[edit | edit source]
Def. any "emission of light that cannot be attributed merely to the temperature of the emitting body"[13] is called luminescence.
Whites
[edit | edit source]
White is the color of fresh milk and snow.[14][15] It is the color the human eye sees when it looks at light which contains all the wavelengths of the visible spectrum, at full brightness and without absorption. It does not have any hue.[16]
"de la couleur de la neige, du lait. Lumiere resultant de la combinaison de toutes les couleurs du spectre solaire."[17] (of the color of snow, of milk. Light resulting from the combination of all the colors of the solar spectrum.)
Blacks
[edit | edit source]

Black is the color of coal, ebony, and of outer space. It is the darkest color, the result of the absence of or complete absorption of light. It is the opposite of white and often represents darkness in contrast with light.[18][19][20]
"Opposite to white: colourless from the absence or complete absorption of light. Also, so near this as to have no distinguishable colour, very dark."[18]
Black is "[t]he darkest color".[19]
"Se dit de la couleur la plus foncée, due à l'absence ou à l'absorption totale des rayons lumineux."[20]("said of the very darkest color, due to the absence or complete absorption of all rays of light.")
Grays
[edit | edit source]


Grey or gray is an intermediate color between black and white, a neutral or achromatic color, meaning literally a color "without color." [21] It is the color of a cloud-covered sky, of ash and of lead.[22]
The first image at right shows some red pebbles among gray pebbles, which are all the same rock type.
The first image at left shows the various shades of grey.
The second image at left of gray clay shows the cracking from water loss.
In the image on the right of a flake of native aluminum, the scale bar = 1 mm.
Aluminium is the third most abundant element (after oxygen and silicon) in the Earth's crust, and the most abundant metal there. It makes up about 8% by mass of the crust, though it is less common in the mantle below.
Violets
[edit | edit source]
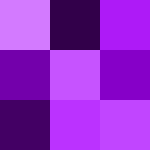








Violet is a bright bluish purple color that takes its name from the violet flower.[19] Violet is at the lower end of the spectrum of light, with a wavelength between approximately 380-450 nanometers.[23]
Def. a bluish-purple colour is called violet.
Def. a colour/color that is a dark blend of red and blue; dark magenta is called purple.
Purple is a range of hues of color occurring between red and blue.[24] The Oxford English Dictionary describes it as a deep, rich shade between crimson and violet.[25]
Axinite-(Mg) or magnesioaxinite, Ca2MgAl2BOSi4O15(OH) magnesium rich, [can be] pale blue to pale violet[26]
Fluorapatite a sample of which is shown at right is a mineral with the formula Ca5(PO4)3F (calcium fluorophosphate). Fluorapatite as a mineral is the most common phosphate mineral. It occurs widely as an accessory mineral in igneous rocks and in calcium rich metamorphic rocks. It commonly occurs as a detrital or diagenic mineral in sedimentary rocks and is an essential component of phosphorite ore deposits. It occurs as a residual mineral in lateritic soils.[27]
At lower left is another fluorapatite example that is violet in color on quartz crystals.
Lower right shows both a rough stone and a cut stone of tanzanite. Tanzanite is the blue/purple variety of the mineral zoisite (a calcium aluminium hydroxy silicate) with the formula (Ca2Al3(SiO4)(Si2O7)O(OH))]. Tanzanite is noted for its remarkably strong trichroism, appearing alternately sapphire blue, violet and burgundy depending on crystal orientation.[28] Tanzanite can also appear differently when viewed under alternate lighting conditions. The blues appear more evident when subjected to fluorescent light and the violet hues can be seen readily when viewed under incandescent illumination. A rough violet sample of tanzanite is third down at left.
Tanzanite in its rough state is usually a reddish brown color. It requires artificial heat treatment to 600 °C in a gemological oven to bring out the blue violet of the stone.[29]
Tanzanite is found only in the foothills of Mount Kilimanjaro.
Tanzanite is universally heat treated in a furnace, with a temperature between 550 and 700 degrees Celsius, to produce a range of hues between bluish-violet to violetish-blue. Some stones found close to the surface in the early days of the discovery were gem-quality blue without the need for heat treatment.
Perhaps the most common violet mineral is sapphire. A sample of uncut natural sapphire is at lowest right. Sapphires may be found naturally, by searching through certain sediments (due to their resistance to being eroded compared to softer stones) or rock formations.
Lepidolite (KLi2Al(Al,Si)3O10(F,OH)2 is a lilac-gray or rose-colored member of the mica group that is a secondary source of lithium. It is a phyllosilicate mineral[30] and a member of the polylithionite-trilithionite series.[31]
It is associated with other lithium-bearing minerals like spodumene in pegmatite bodies. It is one of the major sources of the rare alkali metals rubidium and caesium.[32]
It occurs in granite pegmatites, in some high-temperature quartz veins, greisens and granites. Associated minerals include quartz, feldspar, spodumene, amblygonite, tourmaline, columbite, cassiterite, topaz and beryl.[27]
Blues
[edit | edit source]


Def. of the higher-frequency region of the part of the electromagnetic spectrum which is relevant in the specific observation is called blue.
Def. the colour of the clear sky or the deep sea, between green and violet in the visible spectrum, and one of the primary additive colours for transmitted light; the colour obtained by subtracting red and green from white light using magenta and cyan filters; or any colour resembling this is called blue. Blue is the colour of the clear sky and the deep sea.[33]
"De la couleur du ciel sans nuages, de l'azur"[34]
Sodalite
[edit | edit source]
Sodalite is a rich royal blue mineral. Massive sodalite samples are opaque, crystals are usually transparent to translucent. Occurring typically in massive form, sodalite is found as vein fillings in plutonic igneous rocks such as nepheline syenites.
Covellite
[edit | edit source]
Covellite has been found in veins at depths of 1,150 meters, as the primary mineral. Covellite formed as clusters in these veins reaching one meter across.
Lazurite
[edit | edit source]
Lazurite is a tectosilicate mineral with sulfate, sulfur and chloride with formula: (Na,Ca)8[(S,Cl,SO4,OH)2|(Al6Si6O24)]. It is a feldspathoid and a member of the sodalite group. The colour is due to the presence of S3- anions. Lazurite is a product of contact metamorphism of limestone.
Blueschist facies
[edit | edit source]
| Diagram showing metamorphic facies in pressure-temperature space. The domain of the graph corresponds to circumstances within the Earth's crust and upper mantle. |
A metamorphic facies is a set of metamorphic mineral assemblages that were formed under similar pressures and temperatures.[35] The assemblage is typical of what is formed in conditions corresponding to an area on the two dimensional graph of temperature vs. pressure (See diagram at right).[35] Rocks which contain certain minerals can therefore be linked to certain tectonic settings, times and places in geological history of the area.[35] The boundaries between facies (and corresponding areas on the temperature v. pressure graph), are wide, because they are gradational and approximate.[35] The area on the graph corresponding to rock formation at the lowest values of temperature and pressure, is the range of formation of sedimentary rocks, as opposed to metamorphic rocks, in a process called diagenesis.[35]
Blueschist is a metavolcanic rock that forms by the metamorphism of basalt and rocks with similar composition at high pressures and low temperatures, approximately corresponding to a depth of 15 to 30 kilometers and 200 to ~500 degrees Celsius. The blue color of the rock comes from the presence of the mineral glaucophane. Blueschists are typically found within orogenic belts as terranes of lithology in faulted contact with greenschist or rarely eclogite facies rocks. Blueschist, as a rock type, is defined by the presence of the minerals glaucophane + ( lawsonite or epidote ) +/- jadeite +/- albite or chlorite +/- garnet +/- muscovite in a rock of roughly basaltic composition. Blueschist often has a lepidoblastic, nematoblastic or schistose rock microstructure defined primarily by chlorite, phengitic white mica, glaucophane, and other minerals with an elongate or platy shape. Grain size is rarely coarse, as mineral growth is retarded by the swiftness of the rock's metamorphic trajectory and perhaps more importantly, the low temperatures of metamorphism and in many cases the anhydrous state of the basalts. However, coarse varieties do occur. Blueschists may appear blue, black, gray, or blue-green in outcrop.
Glaucophane
[edit | edit source]
Glaucophane is a mineral belonging to the amphibole group, chemical formula Na2Mg3Al2Si8O22(OH)2 ... The blue color is very diagnostic for this species. It, along with the closely related mineral riebeckite are the only common amphibole minerals that are typically blue. ... Glaucophane forms in metamorphic rocks that are either particularly rich in sodium or that have experienced low temperature-high pressure metamorphism such as would occur along a subduction zone. This material has undergone intense pressure and moderate heat as it was subducted downward toward the mantle. It is glaucophane's color that gives the blueschist facies its name. Glaucophane is also found in eclogites that have undergone retrograde metamorphism.[36]
Hauyne
[edit | edit source]
Hauyne, haüyne or hauynite occurs in Vesuvian lavas in Monte Somma, Italy,[37] It is a tectosilicate mineral with sulfate, with endmember formula Na3Ca(Si3Al3)O12(SO4).[38] It is a feldspathoid and a member of the sodalite group.[39][40] Haüyne occurs in phonolites and related leucite- or nepheline-rich, silica-poor, igneous rocks; less commonly in nepheline-free extrusives[41][39][40][42] and metamorphic rocks (marble).[39]
Water ice
[edit | edit source]
Blue ice occurs when snow falls on a glacier, is compressed, and becomes part of a glacier. Blue ice was observed in Tasman Glacier, New Zealand in January 2011.[43] Ice is blue for the same reason water is blue: it is a result of an overtone of an oxygen-hydrogen (O-H) bond stretch in water which absorbs light at the red end of the visible spectrum.[44]
Cyans
[edit | edit source]




Cyan light has a wavelength of between 490 and 520 nanometers, between the wavelengths of blue and green.[45]
Planck's equation describes the amount of spectral radiance at a certain wavelength radiated by a black body in thermal equilibrium.
In terms of wavelength (λ), Planck's equation is written: as
where B is the spectral radiance, T is the absolute temperature of the black body, kB is the Boltzmann constant, h is the Planck constant, and c is the speed of light.
This form of the equation contains several constants that are usually not subject to variation with wavelength. These are h, c, and kB. They may be represented by simple coefficients: c1 = 2h c2 and c2 = h c/kB.
By setting the first partial derivative of Planck's equation in wavelength form equal to zero, iterative calculations may be used to find pairs of (λ,T) that to some significant digits represent the peak wavelength for a given temperature and vice versa.
Or,
Use c2 = 1.438833 cm K.
For a star to have a peak in the cyan, iterative calculations using the last equation yield the pairs: approximately (476 nm, 6300 K) and (495 nm, 6100 K).
Although Planck's equation is not an exact fit to a star's spectral radiance, it may be close enough to suggest if a star is an astronomical cyan source.
Electric blue is a color close to cyan that is a representation of the color of lightning, an electric spark, and argon signs.
The electric blue glow of electricity results from the spectral emission of the excited ionized atoms (or excited molecules) of air (mostly oxygen and nitrogen) falling back to unexcited states, which happens to produce an abundance of electric blue light. This is the reason electrical sparks in air, including lightning, appear electric blue. It is a coincidence that the color of Cherenkov radiation and light emitted by ionized air are a very similar blue despite their very different methods of production.
Aero blue is a fluorescent cyan color.
The word cerulean is probably derived from the Latin word caeruleus, "dark blue, blue or blue-green", which in turn probably derives from caelulum, diminutive of caelum, "heaven, sky".[46]
Natural gas (methane) has a cyan colored flame when burned with a mixture of air.
The gem-gravel placer deposits of Sri Lanka contain aquamarine.
The deep blue version of aquamarine is called maxixe. Maxixe is commonly found in the country of Madagascar. Its color fades to white when exposed to sunlight or is subjected to heat treatment, though the color returns with irradiation.
The pale blue color of aquamarine is attributed to Fe2+. The Fe3+ ions produce golden-yellow color, and when both Fe2+ and Fe3+ are present, the color is a darker blue as in maxixe. Decoloration of maxixe by light or heat thus may be due to the charge transfer Fe3+ and Fe2+.[47][48][49][50] Dark-blue maxixe color can be produced in green, pink or yellow beryl by irradiating it with high-energy particles (gamma rays, neutrons or even X-rays).[51]
Turquoise at right is an opaque, blue-to-green mineral that is a hydrous phosphate of copper and aluminium, with the chemical formula CuAl6(PO4)4(OH)8·4H2O.
Although fluorite usually appears violet or purple in color, the crystals at left are cyan with some blue or violet fluorite mixed in suggesting slight variations in composition.
Cyan is the color of clear water over a sandy beach, as here at Cala Macaralleta, Menorca.
"Harmful algal blooms, once considered mainly a problem in salt water, have been appearing with increasing severity in the Madison lakes [...] No longer just a smelly, unsightly nuisance, the masses of blue-green algae can also exude toxins that attack the liver or nervous system."[52]
"Given the conditions today, we estimate an 85 percent chance of toxic blue-green algae tomorrow."[53]
"Toxic blue-green algae are also becoming more problematic in many inland waters, including the Great Lakes."[52]
"They are naturally present in lakes that get a lot of nutrients, and are not an invasive species. But we think an increase in phosphorus from the agricultural and urban landscapes is contributing to more severe blooms."[53]
"[S]atellite remote sensing [is being used with] bacteriology and limnology — [to] study [the] lakes".[52]
Greens
[edit | edit source]



Green has a wavelength range of approximately 520–570 nm, a frequency range of ~575–525 THz, with color coordinates of (0, 255, 0) and a hexagonal triplet of #00FF00 from sRGB source of sRGB approximation to NCS S 2060-G.[55]
Def. the colour of growing foliage, as well as other plant cells containing chlorophyll; the colour between yellow and blue in the visible spectrum; one of the primary additive colour for transmitted light; the colour obtained by subtracting red and blue from white light using cyan and yellow filters is called green.
"...in nature chiefly conspicuous as the colour of growing herbage and leaves..."[56]
Green is the color of emeralds, jade, and growing grass.[56] In the continuum of colors of visible light it is located between yellow and blue. Green is the color most commonly associated with nature and the environmental movement, Islam, spring, hope and envy.[57]
Green is the color you see when you look at light with a wavelength of roughly 520–570 nanometers.
It is one of the three additive colors, along with red and blue, which are combined on computer screens and color televisions to make all other colors.
In the subtractive color system, used in printing, it is not a primary color, but is created out of a mixture of yellow and blue, or yellow and cyan.
On the HSV color wheel, also known as the RGB color wheel, the complement of green is magenta; that is, a purple color corresponding to an equal mixture of red and blue light. On a color wheel based on traditional color theory (RYB), the complementary color to green is considered to be red.[58]
The perception of greenness (in opposition to redness forming one of the opponent mechanisms in human color vision) is evoked by light which triggers the medium-wavelength M cone cells in the eye more than the long-wavelength L cones. Light which triggers this greenness response more than the yellowness or blueness of the other color opponent mechanism is called green. A green light source typically has a spectral power distribution dominated by energy with a wavelength of roughly 487–570 nm. More specifically, "blue green" 487–493 nm, "bluish green" 493–498 nm, "green" 498–530 nm, "yellowish green" 530–559 nm, "yellow green" 559–570 nm.[59]
Green earth is a natural pigment ... It s composed of clay colored by iron oxide, magnesium, aluminum silicate, or potassium. Large deposits were found in the South of France near Nice, and in Italy around Verona, on Cyprus, and in Bohemia. The clay was crushed, washed to remove impurities, then powdered. It was sometimes called Green of Verona.[60]
Yellows
[edit | edit source]














"To see day objects with most distinctness, I require a less concave lens by one degree than for seeing the stars best by night, the cause of which seems to be, that the bottom of the eye being illuminated by the day objects, and thereby rendered a light ground, obscures the fainter colours blue indigo and violet in the circle of dissipation, and therefore the best image of the object will be found in the focus of the bright yellow rays, and not in that of the mean refrangible ones, or the dark green, agreeable to Newton's remark, and consequently nearer the retina of a short-sighted person; but the parts of the retina surrounding the circle of dissipation of a star being in the dark, the fainter colours, blue, indigo, and violet, will have some share in forming the image, and consequently the focus will be shorter."[61] Bold added.
"The error due to color loses its disturbing effect because the photographic plate is not sensitive for the red and yellow rays, while the photographically active rays of shorter wave-length are well united by the objective."[62]
"The star brightness increase in 1964 was considerably different in yellow and blue rays. ... Extensive tables and graphs represent the mean photographic and photovisual curve of V1329 Cyg observed in Moscow and Odessa, brightness curves in blue and yellow rays, brightness increases, and brightness minima before and after an outburst."[63]
"The GE Reveal bulb is marketed as the bulb that is made to “specially filter out yellow rays that hide life's true colors.” This is accomplished by the use of neodymium in the glass."[64]
Def. the colour of gold or butter; the colour obtained by mixing green and red light, or by subtracting blue from white light is called yellow.
Def.
a bright yellow colour, resembling the metal gold
is called
| gold. |
Yellow, in the form of yellow ochre pigment made from clay, was one of the first colors used in prehistoric cave art. The cave of Lascaux has an image of a horse colored with yellow estimated to be 17,300 years old.
Shades of yellow contains a more diverse set of yellow or yellow-like colors.
At right is an image of a piece of native gold discovered as part of a placer deposit, a gold nugget.
Sulfur occurs naturally as the pure element (native sulfur) and as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, mentioned for its uses in ancient India, ancient Greece, China and Egypt. Octasulfur is a soft, bright-yellow solid with only a faint odor, similar to that of matches.
Cubanite is a yellow mineral of copper, iron, and sulfur, CuFe2S3.[65] ... Cubanite occurs in high temperature hydrothermal deposits with pyrrhotite and pentlandite as intergrowths with chalcopyrite. It results from exsolution from chalcopyrite at temperatures below 200 to 210 °C.[66] It has also been reported from carbonaceous chondrite meteorites.[66]
Microlite is composed of sodium calcium tantalum oxide with a small amount of fluorine (Na,Ca)2Ta2O6(O,OH,F). Microlite is a mineral in the pyrochlore group that occurs in pegmatites and constitutes an ore of tantalum. It has a Mohs hardness of 5.5 and a variable specific gravity of 4.2 to 6.4. It occurs as disseminated microscopic subtranslucent to opaque octahedral crystals with a refractive index of 2.0 to 2.2. Microlite is also called djalmaite. Microlite occurs as a primary mineral in lithium-bearing granite pegmatites, and in miarolitic cavities in granites.
Orpiment, Arsenic trisulfide As2S3, is a common monoclinic arsenic sulfide mineral. Orpiment is an orange to yellow mineral that is found worldwide [on Earth], and occurs as a sublimation product in volcanic fumaroles, low temperature hydrothermal veins, hot springs and as a byproduct of the decay of another arsenic mineral, realgar.
The mineral pyrite, or iron pyrite, is an iron sulfide with the formula FeS2. This mineral's metallic luster and pale brass-yellow hue have earned it the nickname fool's gold because of its superficial resemblance to gold. Pyrite is the most common of the sulfide minerals [on Earth]. Pyrite is usually found associated with other sulfides or oxides in quartz veins, sedimentary rock, and metamorphic rock, as well as in coal beds, and as a replacement mineral in fossils. Despite being nicknamed fool's gold, pyrite is sometimes found in association with small quantities of gold. Gold and arsenic occur as a coupled substitution in the pyrite structure. In the Carlin–type gold deposits, arsenian pyrite contains up to 0.37 wt% gold.[67]
Satterlyite is a hydroxyl bearing iron phosphate mineral. The mineral can be found in phosphetic shales. Satterlyite is part of the phosphate mineral group. Satterlyite is a transparent, light brown to light yellow mineral. Satterlyite has a formula of (Fe2+,Mg,Fe3+)2(PO4)(OH). Satterlyite occurs in nodules in shale in the Big Fish River (Mandarino, 1978). These nodules were about 10 cm in diameter, some would consist of satterlyite only and others would show satterlyite with quartz, pyrite, wolfeite or maricite.
Holtedahlite, a mineral that was found in Tingelstadtjern quarry in Norway, with the formula (Mg12PO4)5(PO3OH,CO3)(OH,O)6 is isostructural with satterlyite (Raade, 1979). Infrared absorption powder spectra show that satterlyite is different than natural haltedahlite in that there is no carbonate for phosphate substitution (Kolitsch, 2002). Satterlyite is also structurally related to phosphoellenbergerite, a mineral that was discovered in Modum, Norway; near San Giocomo Vallone Di Gilba, in Western Alps of Italy (Palache, 1951); the minerals formula is Mg14(PO4)5(PO3OH)2(OH)6 (Kolitsch, 2002).
Carnotite is a potassium uranium vanadate radioactive mineral with chemical formula: K2(UO2)2(VO4)2·3H2O. The water content can vary and small amounts of calcium, barium, magnesium, iron, and sodium are often present. ... Carnotite is a bright to greenish yellow mineral that occurs typically as crusts and flakes in sandstones. Amounts as low as one percent will color the sandstone a bright yellow. The high uranium content makes carnotite an important uranium ore and also radioactive. It is a secondary vanadium and uranium mineral usually found in sedimentary rocks in arid climates. It is an important ore of uranium in the Colorado Plateau region of the United States where it occurs as disseminations in sandstone and concentrations around petrified logs.
Spurrite is a nesosilicate that can occur naturally as a yellow mineral. Its chemical formula is Ca5(SiO4)2CO3.[68] Spurrite is generally formed in contact metamorphism zones as mafic magmas are intruded into carbonate rocks.[69]
Limonite is an iron ore consisting of a mixture of hydrated iron(III) oxide-hydroxides in varying composition. The generic formula is frequently written as FeO(OH)·nH2O, although this is not entirely accurate as the ratio of oxide to hydroxide can vary quite widely. Limonite is one of the two principle iron ores, the other being hematite, and has been mined for the production of iron since at least 2500 BCE.[70][71] Although originally defined as a single mineral, limonite is now recognized as a mixture of related hydrated iron oxide minerals, among them goethite, akaganeite, lepidocrocite, and jarosite. Individual minerals in limonite may form crystals, but limonite does not, although specimens may show a fibrous or microcrystalline structure,[72] and limonite often occurs in concretionary forms or in compact and earthy masses; sometimes mammillary, botryoidal, reniform or stalactitic. Because of its amorphous nature, and occurrence in hydrated areas limonite often presents as a clay or mudstone. However there are limonite pseudomorphs after other minerals such as pyrite.[73] This means that chemical weathering transforms the crystals of pyrite into limonite by hydrating the molecules, but the external shape of the pyrite crystal remains. Limonite pseudomorphs have also been formed from other iron oxides, hematite and magnetite; from the carbonate siderite and from iron rich silicates such as almandine garnets. ... Limonite usually forms from the hydration of hematite and magnetite, from the oxidation and hydration of iron rich sulfide minerals, and chemical weathering of other iron rich minerals such as olivine, pyroxene, amphibole, and biotite. It is often the major iron component in lateritic soils. One of the first uses was as a pigment. The yellow form produced yellow ochre for which Cyprus was famous,[74]
Oranges
[edit | edit source]























The orange portion of the visible spectrum is from 590 to 620 nm in wavelength.
In optics, orange is the colour seen by the eye when looking at light with a wavelength between approximately 585–620 nm. It has a hue of 30° in HSV colour space.
Def. the colour of a ripe orange (the fruit); a color midway between red and yellow is called orange.
The mineral orpiment [at top right] is a source of yellow and orange pigments. Realgar is an arsenic sulfide mineral of 1.5-2.5 Mohs hardness is used to make red-orange pigment. Crocoite crystals from Dundas extended mine in Tasmania is used to make the first synthetic orange pigment, chrome orange.
Calcite [at second left] is a common calcium carbonate mineral that occurs in orange.
"Taken on Christmas Eve of 2009, this image shows the lower 45 kilometers of the Tsauchab River, a famous landmark for Namibians, tourists, and for orbiting astronauts. The Tsauchab River bed is seen jutting into the sea of red dunes near Namibia’s hyper-arid coast. The riverbed ends in a series of light-colored, silty mud holes on the dry lake floor, known locally as Sossus Vlei (“small lake”)."[76]
Because of the present arid climate, few people have ever seen the Tsauchab River with flowing water or a lake in Sossus Vlei. In times past, however, the Tsauchab appears to have reached the Atlantic coast, another 55 kilometers farther west. Like several other rivers of the coastal Namib Desert, the Tsauchab brings sediment down from the hinterland to the coastal lowland. This sediment is then blown from the river beds, and over probably tens of millions of years, has accumulated as the red dunes of the impressive Namib Sand Sea.
Astronaut photograph ISS022-E-15154 was acquired on December 24, 2009, with a Nikon D2Xs digital camera fitted with an 180 mm lens, and is provided by the ISS Crew Earth Observations experiment and Image Science & Analysis Laboratory, Johnson Space Center. The image was taken by the Expedition 22 crew.
This astronaut photo shows sand heaped up in numerous star dunes, each of them with long arms extending in several directions. Unlike crescent-shaped barchan dunes, which form in areas where winds generally blow from one direction, star dunes are apparently generated where winds are variable. In this part of the Namib Sand Sea, winds are mainly from the south, but easterly winds, channeled along the Tsauchab valley, provide another component. And warm dry winter winds—similar to the Santa Ana winds of California—blow from the northeast.
These northeasterly winds are likely responsible for the regular dune arms that point into the valley from both sides. These large dunes facing the river valley are promoted as the highest dunes in the world. Although continuous dune slopes allow hikers to ascend to altitudes more than 300 meters above the river bottom, not all of that elevation gain has to be walked; the main base of the dunes lies on a terrace 180 meters above the river.
But truly, the now famous sun set at the peak where you will witness the panorama of rolling green hills and also a glimpse of Arabian sea and the bright orange sun going down the hills might force one to contemplate on the nature and its complex beauty. And to witness the same sun rise above the foggy and misty hills and over a blanket of silver clouds the next early morning would be the perfect way to start a day.
At right is an image from LandSat 7 of the Namib Desert. "Namib-Naukluft National Park is an ecological preserve in Namibia's vast Namib Desert. Coastal winds create the tallest sand dunes in the world here, with some dunes reaching 980 feet (300 meters) in height."[77]
"This image was acquired by Landsat 7's Enhanced Thematic Mapper plus (ETM+) sensor on August 12, 2000. This is a false-color composite image made using near infrared, green, and blue wavelengths. The image has also been sharpened using the sensor's panchromatic band."[77]
"Namib-Naukluft National Park [second right] is an ecological preserve in the Namib Desert in southwest Africa, thought to be Earth’s oldest desert. The park is the largest game park in Africa, and a surprising collection of creatures manages to survive in the hyper-arid region, including snakes, geckos, unusual insects, hyenas, and jackals. More moisture comes in as a fog off the Atlantic Ocean than falls as rain, with the average 63 millimeters of rainfall per year concentrated in the months of February and April."[78]
"The winds that bring in the fog are also responsible for creating the park’s towering sand dunes, whose burnt orange color is a sign of their age. The orange color develops over time as iron in the sand is oxidized (like rusty metal); the older the dune, the brighter the color. These dunes are the tallest in the world, in places rising above the desert floor more than 300 meters (almost 1000 feet). The dunes taper off near the coast, and lagoons, wetlands, and mudflats located along the shore attract hundreds of thousands of birds."[78]
The Namib is a coastal desert in southern Africa.
Southern Namib (between Lüderitz and the Kuiseb River) comprises a vast dune sea with some of the tallest and most spectacular dunes of the world, ranging in color from pink to vivid orange. In the Sossusvlei area, several dunes exceed 300 meters (984 ft) in height. The complexity and regularity of dune patterns in its dune sea have attracted the attention of geologists for decades, but it remains poorly understood.
The Namib-Naukluft National Park, that extends over a large part of the Namib Desert, is the largest game reserve in Africa and one of the largest of the world. While most of the park is hardly accessible, several well-known visitor attractions are found in the desert. The prominent attraction is the famous Sossusvlei area, where high orange sand dunes surround vivid white salt pans, creating a fascinating landscape.
The Sossusvlei area belongs to a wider region of southern Namib with homogeneous features (about 32.000 km²) extending between rivers Koichab and Kuiseb. This area is characterized by high sand dunes of vivid pink-to-orange color, a consequence of a high percentage of iron in the sand and consequent oxidation processes. The oldest dunes are those of a more intense reddish color. These dunes are among the highest in the world; many of them are above 200 metres, the highest being the one nicknamed Big Daddy, about 380 metres high.
Sossusvlei is about 66 km past the Sesriem gate. The last 6 km can only be traversed with 4WD vehicles as the concrete road ends and sand begins (the place where the concrete road ends is known as "2x4 parking" as any non-4WD vehicle must stop there). Sossusvlei is a clay pan, of roughly elliptical shape, covered in a crust of salt-rich sand.[79] While the pan has been shaped over time by the Tsauchab river, the actual flooding of the pan is a relatively rare event, and sometimes several years pass between one flood and the next one. The river is dry most of the year, and even when it is not, it carries relatively little water to the vlei. The vlei is surrounded by high orange-reddish dunes, partially covered by a vegetation comprising grass, bushes, and some tree (mostly of species Acacia erioloba).
Deadvlei is another clay pan, about 2 km from Sossusvlei. A notable feature of Deadvlei is that it used to be an oasis with several acacia trees; afterwards, the river that watered the oasis changed its course. The pan is thus punctuated by blackened, dead acacia trees, in vivid contrast to the shiny white of the salty floor of the pan and the intense orange of the dunes. This creates a particularly fascinating and surrealistic landscape, that appears in uncountable pictures and that has been used as a setting for films and videos.
"The striking orange-red colored southern Australian coast contrasts against the deep sapphire-blue waters of the Southern Ocean in this true-color Moderate Resolution Imaging Spectroradiometer (MODIS) image acquired by the Terra satellite on August 19, 2002. In the northern portion of the image, a handful of fires (marked in red) were detected burning in the Great Victoria Desert by the MODIS instrument. South of the desert is the lighter-orange Nullarbor Plain, which stretches for over 1000 kilometers (about 600 miles) from end to end."[80]
Reds
[edit | edit source]










In wavelengths, red astronomy covers 620 - 750 nm.
Far-red light is light at the extreme red end of the visible spectrum, between red and infra-red light. Usually regarded as the region between 710 and 850 nm wavelength, it is dimly visible to some [human] eyes.
Infrared or red radiation from a common household radiator or electric heater is an example of thermal radiation, as is the heat emitted by an operating incandescent light bulb. Thermal radiation is generated when energy from the movement of charged particles within atoms is converted to electromagnetic radiation.
Infrared (IR) light is electromagnetic radiation with longer wavelengths than those of visible light, extending from the nominal red edge of the visible spectrum at 700 nanometres (nm) to 1 mm. This range of wavelengths corresponds to a frequency range of approximately 430 THz down to 300 GHz,[81] and includes most of the thermal radiation emitted by objects near room temperature. Infrared light is emitted or absorbed by molecules when they change their rotational-vibrational movements.
Rhodolite is a varietal name for rose-pink to red mineral pyrope, a species in the garnet group.
Chemically, rhodolite is an iron-magnesium-aluminium silicate, [(Mg,Fe)3Al2(SiO4)3,] part of the pyrope-almandine solid-solution series, with an approximate garnet composition of Py70Al30.
Breithauptite is a nickel antimonide mineral with the simple formula NiSb. Breithauptite is a metallic opaque copper-red mineral crystallizing in the hexagonal - dihexagonal dipyramidal crystal system. It is typically massive to reniform in habit, but is observed as tabular crystals. It has a Mohs hardness of 3.5 to 4 and a specific gravity of 8.23.
It occurs in hydrothermal calcite veins associated with cobalt–nickel–silver ores.
Cinnabar ... or cinnabarite ... (red mercury(II) sulfide (HgS), native vermilion), is the common ore of mercury. [Its color is cochineal-red, towards brownish red and lead-gray] ... Cinnabar [may be] found in a massive, granular or earthy form and is bright scarlet to brick-red in color.[82] ... Generally cinnabar occurs as a vein-filling mineral associated with recent volcanic activity and alkaline hot springs. Cinnabar is deposited by epithermal ascending aqueous solutions (those near surface and not too hot) far removed from their igneous source.
Crocoite is a mineral consisting of lead chromate, PbCrO4. Crystals are of a bright hyacinth-red color. Relative rarity of crocoite is connected with specific conditions required for its formation: an oxidation zone of lead ore bed and presence of ultramafic rocks serving as the source of chromium (in chromite).
Eudialyte ... is a somewhat rare, red silicate mineral, which forms in alkaline igneous rocks, such as nepheline syenites.
Hematite is the mineral form of iron(III) oxide (Fe2O3), one of several iron oxides. Hematite is colored black to steel or silver-gray, brown to reddish brown, or red. Huge deposits of hematite are found in banded iron formations.
The Belt of Venus or Venus's Girdle is the Victorian-era name for an atmospheric phenomenon seen at sunrise and sunset. Shortly after sunset or shortly before sunrise, the observer is, or is very nearly, surrounded by a pinkish glow (or anti-twilight arch) that extends roughly 10°–20° above the horizon. Often, the glow is separated from the horizon by a dark layer, the Earth's shadow or "dark segment". The Arch's light rose (pink) color is due to backscattering of reddened light from the rising or setting Sun.
The first image at left shows the Belt of Venus from 42,000 feet altitude locally above the Earth's surface.
In the image at right is the Belt of Venus, a pink band that is visible above the dark blue of the Earth's shadow, in the same part of the sky. No defined line divides the Earth's shadow and the Belt of Venus; one colored band blends into the other in the sky.
In the image at second left, the Belt of Venus is shown at sunset, looking east from the Marin Headlands just north of San Francisco. There is a thin greyish cloud layer partially obscuring the horizon in this image.
The second image at right shows the full Moon rising as seen through the Belt of Venus.
In the right viewing conditions, a pink (or orange or purple) band is visible in the twilight sky just above the dark blue band of the Earth's shadow. This pink band is called the "anti-twilight arch" or "Belt of Venus". The name "Belt of Venus" is not connected with the planet Venus; the Belt of Venus is part of Earth's upper atmosphere which is illuminated by the setting or rising sun. It is visible either after the sun ceases to be visible (at sunset) or before the sun becomes visible (at sunrise).[83][84]
When the sun is near the horizon at sunset or sunrise, the light from the sun is red; this is because the light is reaching the observer through an especially thick layer of the atmosphere, which works as a filter, scattering all but the red light.
From the viewpoint of the observer, the red sunlight directly illuminates small particles in the lower atmosphere on the other side of the sky from the sun. The red light is backscattered to the observer, and that is why the Belt of Venus appears pink.
The lower the sunset sun descends, the less clearly distinguished the boundary between the Earth's shadow and Belt of Venus becomes. This is because now the setting sun illuminates a thinner part of the upper atmosphere. The red light is not scattered there because there are fewer particles, and the eye only sees the "normal" (usual) blue sky, which is due to Rayleigh scattering from air molecules. Eventually, both the Earth's shadow and the Belt of Venus dissolve into the darkness of the night sky.
Infrareds
[edit | edit source]The wavelength of infrared light ranges from 0.75 to 300 micrometers. Infrared falls in between visible radiation, which ranges from 380 to 750 nanometers, and submillimeter waves. Infrared rays can be emitted, fluoresced, or reflected by an astronomical object.
Def. electromagnetic radiation of a wavelength longer than visible light, but shorter than microwave radiation, having a wavelength between 700 nm and 1 mm is called infrared.
Def. [e]lectromagnetic radiation having a wavelength approximately between 1 micrometre and 1 millimetre; perceived as heat is called infrared radiation.
Astronomers typically divide the infrared spectrum as follows:[85]
| Designation | Abbreviation | Wavelength |
|---|---|---|
| Near Infrared | NIR | (0.7–1) to 5 µm |
| Mid Infrared | MIR | 5 to (25–40) µm |
| Far Infrared | FIR | (25–40) to (200–350) µm. |
These are the approximate ranges for photon energies of the infrared bands:
| Division Name | Wavelength | Photon Energy |
| Near-infrared | 0.75-1.4 µm | 0.9-1.7 eV |
| Short-wavelength infrared | 1.4-3 µm | 0.4-0.9 eV |
| Mid-wavelength infrared | 3-8 µm | 150-400 meV |
| Long-wavelength infrared | 8–15 µm | 80-150 meV |
| Far infrared | 15 - 1,000 µm | 1.2-80 meV |
| Wavelength range (micrometres) |
Astronomical bands | Telescopes |
|---|---|---|
| 0.65 to 1.0 | R and I bands | All major optical telescopes |
| 1.1 to 1.4 | J band | Most major optical telescopes and most dedicated infrared telescopes |
| 1.5 to 1.8 | H band | Most major optical telescopes and most dedicated infrared telescopes |
| 2.0 to 2.4 | K band | Most major optical telescopes and most dedicated infrared telescopes |
| 3.0 to 4.0 | L band | Most dedicated infrared telescopes and some optical telescopes |
| 4.6 to 5.0 | M band | Most dedicated infrared telescopes and some optical telescopes |
| 7.5 to 14.5 | N band | Most dedicated infrared telescopes and some optical telescopes |
| 17 to 25 | Q band | Some dedicated infrared telescopes and some optical telescopes |
| 28 to 40 | Z band | Some dedicated infrared telescopes and some optical telescopes |
| 330 to 370 | Some dedicated infrared telescopes and some optical telescopes | |
| 450 | submillimeter | Submillimeter telescopes |
The infrared band may be divided up “based on the response of various detectors:[86]”
- Near infrared: from 0.7 to 1.0 µm (from the approximate end of the response of the human eye to that of silicon).
- Short-wave infrared: 1.0 to 3 µm (from the cut off of silicon to that of the MWIR atmospheric window. InGaAs covers to about 1.8 µm; the less sensitive lead salts cover this region.
- Mid-wave infrared: 3 to 5 µm (defined by the atmospheric window and covered by indium antimonide [InSb] and HgCdTe and partially by lead selenide [PbSe]).
- Long-wave infrared: 8 to 12, or 7 to 14 µm: the atmospheric window (Covered by HgCdTe and microbolometers).
- Very-long wave infrared (VLWIR): 12 to about 30 µm, covered by doped silicon.
A commonly used sub-division scheme is:[87]
| Division Name | Abbreviation | Wavelength | Characteristics |
| Near-infrared | NIR, IR-A DIN | 0.75-1.4 µm | Defined by the water absorption, and commonly used in fiber optic telecommunication because of low attenuation losses in the SiO2 glass (silica) medium. Image intensifiers are sensitive to this area of the spectrum. Examples include night vision devices such as night vision goggles. |
| Short-wavelength infrared | SWIR, IR-B DIN | 1.4-3 µm | Water absorption increases significantly at 1,450 nm. The 1,530 to 1,560 nm range is the dominant spectral region for long-distance telecommunications. |
| Mid-wavelength infrared | MWIR, IR-C DIN. Also called intermediate infrared (IIR) | 3-8 µm | In guided missile technology the 3-5 µm portion of this band is the atmospheric window in which the homing heads of passive IR 'heat seeking' missiles are designed to work, homing on to the Infrared signature of the target aircraft, typically the jet engine exhaust plume |
| Long-wavelength infrared | LWIR, IR-C DIN | 8–15 µm | This is the "thermal imaging" region, in which sensors can obtain a completely passive picture of the outside world based on thermal emissions only and requiring no external light or thermal source such as the sun, moon or infrared illuminator. Forward-looking infrared (FLIR) systems use this area of the spectrum. This region is also called the "thermal infrared." |
| Far infrared | FIR | 15 - 1,000 µm | (see also far-infrared laser). |
NIR and SWIR is sometimes called "reflected infrared" while MWIR and LWIR is sometimes referred to as "thermal infrared." Due to the nature of the blackbody radiation curves, typical 'hot' objects, such as exhaust pipes, often appear brighter in the MW compared to the same object viewed in the LW.
Submillimeters
[edit | edit source]
"[T]erahertz radiation refers to electromagnetic waves propagating at frequencies in the terahertz range. It is synonymously termed submillimeter radiation, terahertz waves, terahertz light, T-rays, T-waves, T-light, T-lux, THz. The term typically applies to electromagnetic radiation with frequencies between high-frequency edge of the microwave band, 300 gigahertz (3 x 1011 Hz),"[88] and the long-wavelength edge of far-infrared light, 3000 GHz (3 x 1012 Hz or 3 THz). In wavelengths, this range corresponds to 0.1 mm (or 100 µm) infrared to 1.0 mm microwave.
Terahertz radiation is emitted as part of the black body radiation from anything with temperatures greater than about 10 kelvin. While this thermal emission is very weak, observations at these frequencies are important for characterizing the cold 10-20K dust in the interstellar medium in the Milky Way galaxy, and in distant starburst galaxies. Telescopes operating in this band include the James Clerk Maxwell Telescope, the Caltech Submillimeter Observatory and the Submillimeter Array at the Mauna Kea Observatory in Hawaii, the BLAST balloon borne telescope, the Herschel Space Observatory, and the Heinrich Hertz Submillimeter Telescope at the Mount Graham International Observatory in Arizona. The Atacama Large Millimeter Array, under construction, will operate in the submillimeter range. The opacity of the Earth's atmosphere to submillimeter radiation restricts these observatories to very high altitude sites, or to space.
Microwaves
[edit | edit source]Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz (0.3 GHz) and 300 GHz.[89] This broad definition includes both [ultra high frequency] UHF and [extremely high frequency] EHF (millimeter waves), and various sources use different boundaries.[90] In all cases, microwave includes the entire [super high frequency] SHF band (3 to 30 GHz, or 10 to 1 cm) at minimum, with [radio frequency] RF engineering often putting the lower boundary at 1 GHz (30 cm), and the upper around 100 GHz (3 mm).
| Letter Designation | Frequency range | Wavelength range | Typical uses |
|---|---|---|---|
| L band | 1 to 2 GHz | 15 cm to 30 cm | military telemetry, GPS, mobile phones (GSM), amateur radio |
| S band | 2 to 4 GHz | 7.5 cm to 15 cm | weather radar, surface ship radar, and some communications satellites (microwave ovens, microwave devices/communications, radio astronomy, mobile phones, wireless LAN, Bluetooth, ZigBee, GPS, amateur radio) |
| C band | 4 to 8 GHz | 3.75 cm to 7.5 cm | long-distance radio telecommunications |
| X band | 8 to 12 GHz | 25 mm to 37.5 mm | satellite communications, radar, terrestrial broadband, space communications, amateur radio |
| Ku band or P band | 12 to 18 GHz | 16.7 mm to 25 mm | satellite communications |
| K band | 18 to 26.5 GHz | 11.3 mm to 16.7 mm | radar, satellite communications, astronomical observations, automotive radar |
| Ka band | 26.5 to 40 GHz | 5.0 mm to 11.3 mm | satellite communications |
| Q band | 33 to 50 GHz | 6.0 mm to 9.0 mm | satellite communications, terrestrial microwave communications, radio astronomy, automotive radar |
| U band | 40 to 60 GHz | 5.0 mm to 7.5 mm | |
| V band | 50 to 75 GHz | 4.0 mm to 6.0 mm | millimeter wave radar research and other kinds of scientific research |
| W band | 75 to 110 GHz | 2.7 mm to 4.0 mm | satellite communications, millimeter-wave radar research, military radar targeting and tracking applications, and some non-military applications, automotive radar |
| F band | 90 to 140 GHz | 2.1 mm to 3.3 mm | SHF transmissions: Radio astronomy, microwave devices/communications, wireless LAN, most modern radars, communications satellites, satellite television broadcasting, DBS, amateur radio |
| D band | 110 to 170 GHz | 1.8 mm to 2.7 mm | EHF transmissions: Radio astronomy, high-frequency microwave radio relay, microwave remote sensing, amateur radio, directed-energy weapon, millimeter wave scanner |
"Many natural sources, besides salinity, contribute to the microwave radiation at L-band frequencies (approximately 1 gigahertz) measured by satellites. Correcting the influence of these natural sources is a key to obtaining Aquarius’ accurate salinity measurements."[91]
Radars
[edit | edit source]
Radio waves are a type of electromagnetic radiation with wavelengths in the electromagnetic spectrum longer than infrared light. Radio waves have frequencies from 300 [Gigahertz] GHz to as low as 3 [Kilohertz] kHz, and corresponding [to] wavelengths from 1 millimeter to 100 kilometers.
| Band name | Frequency range | Wavelength range | Notes |
|---|---|---|---|
| HF | 3–30 MHz | 10–100 m | Coastal radar systems, over-the-horizon radar (OTH) radars; 'high frequency' |
| VHF | 30–300 MHz | 1–10 m | Very long range, ground penetrating; 'very high frequency' |
| P | < 300 MHz | > 1 m | 'P' for 'previous', applied retrospectively to early radar systems; essentially HF + VHF |
| UHF | 300–1000 MHz | 0.3–1 m | Very long range (e.g. ballistic missile early warning), ground penetrating, foliage penetrating; 'ultra high frequency' |
| L | 1–2 GHz | 15–30 cm | Long range air traffic control and surveillance; 'L' for 'long' |
| S | 2–4 GHz | 7.5–15 cm | Moderate range surveillance, Terminal air traffic control, long-range weather, marine radar; 'S' for 'short' |
| C | 4–8 GHz | 3.75–7.5 cm | Satellite transponders; a compromise (hence 'C') between X and S bands; weather; long range tracking |
| X | 8–12 GHz | 2.5–3.75 cm | Missile guidance, marine radar, weather, medium-resolution mapping and ground surveillance; in the USA the narrow range 10.525 GHz ±25 MHz is used for airport radar; short range tracking. Named X band because the frequency was a secret during WW2. |
| Ku | 12–18 GHz | 1.67–2.5 cm | High-resolution, also used for satellite transponders, frequency under K band (hence 'u') |
| K | 18–24 GHz | 1.11–1.67 cm | From German kurz, meaning 'short'; limited use due to absorption by water vapour, so Ku and Ka were used instead for surveillance. K-band is used for detecting clouds by meteorologists, and by police for detecting speeding motorists. K-band radar guns operate at 24.150 ± 0.100 GHz. |
| Ka | 24–40 GHz | 0.75–1.11 cm | Mapping, short range, airport surveillance; frequency just above K band (hence 'a') Photo radar, used to trigger cameras which take pictures of license plates of cars running red lights, operates at 34.300 ± 0.100 GHz. |
| mm | 40–300 GHz | 1.0–7.5 mm | Millimetre band, subdivided as below. The frequency ranges depend on waveguide size. Multiple letters are assigned to these bands by different groups. These are from Baytron, a now defunct company that made test equipment. |
| V | 40–75 GHz | 4.0–7.5 mm | Very strongly absorbed by atmospheric oxygen, which resonates at 60 GHz. |
| W | 75–110 GHz | 2.7–4.0 mm | Used as a visual sensor for experimental autonomous vehicles, high-resolution meteorological observation, and imaging. |
Numerous airborne and spacecraft radars have mapped the entire planet, for various purposes. One example is the Shuttle Radar Topography Mission, which mapped the entire Earth at 30 m resolution.
At right is a shaded relief map of Antarctica developed from RADARSAT Synthetic Aperture Radar data. RADARSAT is a Canadian satellite.
Radios
[edit | edit source]Radio waves are a type of electromagnetic radiation with wavelengths in the electromagnetic spectrum longer than infrared light. Radio waves have frequencies from 300 Gigahertz (GHz) to as low as 3 Kilohertz (kHz), and corresponding wavelengths from 1 millimeter to 100 kilometers.
See also
[edit | edit source]References
[edit | edit source]- ↑ C. L. Bhat (December 1997). "Ground-based γ-ray astronomy : Present status and future prospects". Bulletin of the Astronomical Society of India 25 (12): 461-84.
- ↑ Trent J. Perrotto (10 January 2012). NASA's Fermi Space Telescope Explores New Energy Extremes. Washington, DC USA: NASA. http://www.nasa.gov/mission_pages/GLAST/news/energy-extremes.html. Retrieved 2016-11-03.
- ↑ Wyatt McNally (31 October 2013). Thorium and the Liquid-Fluoride Thorium Reactor Concept. Slideplayer. http://slideplayer.com/slide/763101/. Retrieved 2016-11-12.
- ↑ 4.0 4.1 Mari-Anne Hedblad; Lotus Mallbris (July 2012). "Grenz ray treatment of lentigo maligna and early lentigo maligna melanoma". Journal of the American Academy of Dermatology 67 (1): 60-8. doi:10.1016/j.jaad.2011.06.029. http://www.jaad.org/article/S0190-9622%2811%2900837-1/abstract. Retrieved 2016-09-22.
- ↑ R. C. Bless; A. D. Code (1972). "Ultraviolet Astronomy". Annual Review of Astronomy and Astrophysics 10: 197-226. doi:10.1146/annurev.aa.10.090172.001213.
- ↑ McGraw-Hill Encyclopedia of Science and Technology (5th ed.). McGraw-Hill 1993.
- ↑ Moore, P.. Philip's Atlas of the Universe 1997. Great Britain: George Philis Limited. ISBN 0-540-07465-9.
- ↑ Willard Lincoln Roberts; George Robert Rapp Jr.; Julius Weber (1974). Encyclopedia of Minerals. New York, New York, USA: Van Nostrand Reinhold Company. pp. 693. ISBN 0-442-26820-3.
- ↑ Schmid, H. M.; Beuzit, J.-L.; Feldt, M. (2006). "Search and investigation of extra-solar planets with polarimetry". Direct Imaging of Exoplanets: Science & Techniques. Proceedings of the IAU Colloquium #200 1 (C200): 165–170. doi:10.1017/S1743921306009252.
- ↑ Cecie Starr (2005). Biology: Concepts and Applications. Thomson Brooks/Cole. ISBN 053446226X. http://books.google.com/?id=RtSpGV_Pl_0C&pg=PA94.
- ↑ Principles Of Instrumental Analysis F.James Holler, Douglas A. Skoog & Stanley R. Crouch 2006
- ↑ Tom Harris. How Fluorescent Lamps Work. Discovery Communications. http://home.howstuffworks.com/fluorescent-lamp.htm. Retrieved 27 June 2010.
- ↑ SemperBlotto (3 April 2006). luminescence. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/luminescence. Retrieved 2016-09-22.
- ↑ Shorter Oxford English Dictionary, 5th Edition (2002)
- ↑ See Shorter Oxford English Dictionary, 5th Edition (2002); "of the colour of fresh milk or snow." See also Webster's New World Dictionary of American English, Third College Edition, (1988): "Having the color of pure snow or milk." See also The Random House College Dictionary of the English Language, Revised Edition,(1980)
- ↑ Shorter Oxford English Dictionary, 5th Edition (2002)
- ↑ Petit Larousse (2005).
- ↑ 18.0 18.1 Shorter Oxford English Dictionary. 5th Edition (2002)
- ↑ 19.0 19.1 19.2 See also Webster's New World Dictionary of the American Language (1964)
- ↑ 20.0 20.1 Le Petit Larousse Illustré (1997)
- ↑ Webster's New World Dictionary of the American Language, Third College Edition.
- ↑ Shorter Oxford English Dictionary, 5th edition, 2002.
- ↑ J. W. G. Hunt (1980). Measuring Color. Ellis Horwood Ltd. ISBN 0-7458-0125-0.
- ↑ Mish, Frederic C., Editor in Chief Webster's Ninth New Collegiate Dictionary Springfield, Massachusetts, U.S.A.:1984--Merriam-Webster Page 957
- ↑ Shorter Oxford English Dictionary, 5th Edition, 2003.
- ↑ Handbook of Mineralogy: Magnesioaxinite
- ↑ 27.0 27.1 http://rruff.geo.arizona.edu/doclib/hom/fluorapatite.pdf Mineral Handbook
- ↑ E. Skalwold. Pleochroism: trichroism and dichroism in gems. Nordskip.com. http://www.nordskip.com/pleochroism.html. Retrieved 2011-08-29.
- ↑ YourGemologist / International School of Gemology Study of Heat Treatment. Yourgemologist.com. http://www.yourgemologist.com/heattreatment.html. Retrieved 2011-08-29.
- ↑ Hurlbut, Cornelius S.; Klein, Cornelis (1985), Manual of Mineralogy, Wiley, (20th ed.) ISBN 0-471-80580-7
- ↑ Lepidolite on Mindat.org
- ↑ H. Nechamkin, The Chemistry of the Elements, McGraw-Hill, New York, 1968.
- ↑ See Webster's New World Dictionary of the American Language, College Edition, 1962.
- ↑ Le Petit Larousse illustré 1997. Librairie Larousse. 1996. pp. 1784. ISBN 2033011976. http://books.google.com/books?id=ZWSemgEACAAJ&hl=en&sa=X&ei=P72mUauzBoba9ASxhYHoAQ&ved=0CFQQ6AEwDw. Retrieved 2013-05-28.
- ↑ 35.0 35.1 35.2 35.3 35.4 Essentials of Geology, 3rd Edition, Stephen Marshak
- ↑ http://rruff.geo.arizona.edu/doclib/hom/glaucophane.pdf Handbook of Mineralogy
- ↑ Farndon and Parker (2009). Minerals, Rocks and Fossils of the World. Lorenz Books
- ↑ http://rruff.info/ima
- ↑ 39.0 39.1 39.2 Gaines (1997). Dana’s New Mineralogy Eighth Edition. New York: Wiley.
- ↑ 40.0 40.1 Hauyne. Mindat.org. http://www.mindat.org/min-1833.html. Retrieved 11 August 2011.
- ↑ Hauyne. Webminerals. http://webmineral.com/data/Hauyne.shtml. Retrieved 11 August 2011.
- ↑ Handbook of Mineralogy
- ↑ Harvey, Eveline (14 January 2011). NZ blue ice sighting an unexpected treat for tourists. http://www.nzherald.co.nz/travel/news/article.cfm?c_id=7&objectid=10699700. Retrieved 21 September 2011.
- ↑ Why Is Water Blue
- ↑ About.com - Physics About.Com - Physics (Retrieved 6-18-2013)
- ↑ Cerulean, Online Etymology Dictionary
- ↑ Color in the Beryl group. http://minerals.caltech.edu/FILES/Visible/BERYL/Index.htm. Retrieved 2009-06-06.
- ↑ Ibragimova E. M.; Mukhamedshina N. M.; Islamov A. Kh. (2009). "Correlations between admixtures and color centers created upon irradiation of natural beryl crystals". Inorganic Materials 45 (2): 162. doi:10.1134/S0020168509020101.
- ↑ Viana R. R.; Da Costa G. M.; De Grave E.; Stern W. B.; Jordt-Evangelista H.. "Characterization of beryl (aquamarine variety) by Mössbauer spectroscopy 2002". Physics and Chemistry of Minerals 29: 78. doi:10.1007/s002690100210.
- ↑ Blak, Ana Regina; Isotani, Sadao; Watanabe, Shigueo. "Optical absorption and electron spin resonance in blue and green natural beryl: A reply 1983". Physics and Chemistry of Minerals 9 (6): 279. doi:10.1007/BF00309581.
- ↑ K. Nassau (1976). "The deep blue Maxixe-type color center in beryl". American Mineralogist 61: 100. http://www.minsocam.org/ammin/AM61/AM61_100.pdf.
- ↑ 52.0 52.1 52.2 David Tenenbaum (July 1, 2010). Confronting toxic blue-green algae in Madison lakes. PhysOrg. http://phys.org/news197212057.html. Retrieved 2014-02-24.
- ↑ 53.0 53.1 Katherine McMahon (July 1, 2010). Confronting toxic blue-green algae in Madison lakes. PhysOrg. http://phys.org/news197212057.html. Retrieved 2014-02-24.
- ↑ Malachite. WebExhibits. 2001. http://webexhibits.org/pigments/indiv/overview/malachite.html. Retrieved 2007-12-08.
- ↑ The sRGB values are taken by converting the NCS color 2060-G using the "NCS Navigator" tool at the NCS website.
- ↑ 56.0 56.1 (Oxford English Dictionary, 2nd Edition, Clarendon Press, Oxford, 1989.) See also first definition in Webster's New World Dictionary of the American Language, The World Publishing Company, New York, 1962.
- ↑ Eva Heller, Psychologie de la couleur- effets et symboliques. pg. 87-104
- ↑ Glossary Term: Color wheel. Sanford Corp.. 2005. http://web.archive.org/web/20071102211428/http://www.sanford-artedventures.com/study/g_color_wheel.html. Retrieved 2007-11-22.
- ↑ Kenneth L. Kelly (1943). "Color Designations for Lights". Journal of the Optical Society of America 33(11). 627–632.
- ↑ Anne Varichon (2000), Couleurs- pigments et teintures dans les mains des peoples. Pg. 210-211.
- ↑ Nevil Maskelyne (June 18 1789). "An Attempt to Explain a Difficulty in the Theory of Vision, Depending on the Different Refrangibility of Light". Philosophical Transactions of the Royal Society of London 79: 256-64. http://www.jstor.org/stable/106696. Retrieved 2013-09-13.
- ↑ J. Hartmann (May 1908). "An Improvement of the Foucault Knife-Edge Test in the Investigation of Telescope Objectives". The Astrophysical Journal 27: 254-9. doi:10.1086/141552. http://adsabs.harvard.edu/abs/1908ApJ....27..254H. Retrieved 2013-09-13.
- ↑ V. P. Arkhipova; O. E. Mandel (April 1991). "Photographic observations of V1329 CYG". Pis'ma v Astronomicheskii Zhurnal 17 (04): 359-67. http://adsabs.harvard.edu/abs/1991PAZh...17..359A. Retrieved 2013-09-13.
- ↑ Jennifer J. Birriel (September 2008). "Demonstrating Absorption Spectra Using Commercially Available Incandescent Light Bulbs". Astronomy Education Review 7 (2): 147-57. doi:10.3847/AER2008035. http://link.aip.org/link/?AERSCZ/7/147/1. Retrieved 2013-09-13.
- ↑ Webmineral
- ↑ 66.0 66.1 Handbook of Mineralogy
- ↑ M. E. Fleet and A. Hamid Mumin, Gold-bearing arsenian pyrite and marcasite and arsenopyrite from Carlin Trend gold deposits and laboratory synthesis, American Mineralogist 82 (1997) pp. 182–193
- ↑ Richard V. Gaines, H. Catherine W. Skinner, Eugene E. Foord, Brian Mason, and Abraham Rosenzweig: Dana's new mineralogy, p. 1106. John Wiley & Sons, 1997
- ↑ Smith, J.V. (1960) "The Crystal structure of Spurrite, Ca5(SiO4)2CO3". Acta. Cryst. 13, 454
- ↑ MacEachern, Scott (1996) "Iron Age beginnings north of the Mandara Mountains, Cameroon and Nigeria" pp. 489–496 In Pwiti, Gilbert and Soper, Robert (editors) (1996) Aspects of African Archaeology: Proceedings of the Tenth Pan-African Congress University of Zimbabwe Press, Harare, Zimbabwe, ISBN 978-0-908307-55-5; archived here by Internet Archive on 11 March 2012
- ↑ Diop-Maes, Louise Marie (1996) "La question de l'Âge du fer en Afrique" ("The question of the Iron Age in Africa") Ankh 4/5: pp. 278–303, in French; archived here by Internet Archive on 25 January 2008
- ↑ Boswell, P. F. and Blanchard, Roland (1929) "Cellular structure in limonite" Economic Geology 24(8): pp. 791–796
- ↑ Northrop, Stuart A. (1959) "Limonite" Minerals of New Mexico (revised edition) University of New Mexico Press, Albuquerque, New Mexico, pp. 329–333 }}
- ↑ Constantinou, G. and Govett, G. J. S. (1972) "Genesis of sulphide deposits, ochre and umber of Cyprus" Transactions of the Institution of Mining and Metallurgy 81: pp. 34–46
- ↑ Andrew Goudie (2010). Piotr Migoń. ed. Chapter 17: Namib Sand Sea: Large Dunes in an Ancient Desert, In: Geomorphological Landscapes of the World. New York, NY: Springer. pp. 163–169. ISBN 978-90-481-3054-2. http://books.google.com/books?id=-TI55urJYyEC&lpg=PR2&pg=PR2#v=onepage&q&f=false.
- ↑ Astronaut photograph (December 24, 2009). TSAUCHAB RIVER AND SOSSUS VLEI LAKEBED, NAMIBIA. Washington, DC USA: NASA. http://visibleearth.nasa.gov/view.php?id=42396. Retrieved 2014-03-04.
- ↑ 77.0 77.1 Charles Ichoku (September 23, 2002). Namib-Naukluft National Park, Namibia. Washington, DC USA: NASA. http://earthobservatory.nasa.gov/IOTD/view.php?id=2804&src=ve. Retrieved 2014-03-04.
- ↑ 78.0 78.1 JAROS (October 11, 2003). High Dunes in the Namib Desert. Washington, DC USA: Earth Observatory, NASA. http://earthobservatory.nasa.gov/IOTD/view.php?id=3866&eocn=image&eoci=related_image. Retrieved 2014-03-04.
- ↑ Deadvlei. Atlasobscura.com. http://atlasobscura.com/place/dead-vlei. Retrieved 2012-12-26.
- ↑ Jacques Descloitres (August 19, 2002). Australian South Coast. Washington, DC USA: NASA. http://visibleearth.nasa.gov/view.php?id=61798. Retrieved 2014-03-04.
- ↑ S. C. Liew. Electromagnetic Waves. Centre for Remote Imaging, Sensing and Processing. http://www.crisp.nus.edu.sg/~research/tutorial/em.htm. Retrieved 2006-10-27.
- ↑ R. J. King (2002). "Minerals Explained 37: Cinnabar". Geology Today 18 (5): 195–9. doi:10.1046/j.0266-6979.2003.00366.x.
- ↑ Les Cowley. Earth's shadow. www.atoptics.co.uk. http://www.atoptics.co.uk/atoptics/earshad.htm.
- ↑ What causes layers in the sunrise and sunset?. earthsky.org. http://www.webexhibits.org/causesofcolor/14E.html.
- ↑ IPAC Staff. Near, Mid and Far-Infrared. NASA ipac. http://www.ipac.caltech.edu/Outreach/Edu/Regions/irregions.html. Retrieved 2007-04-04.
- ↑ Miller, Principles of Infrared Technology (Van Nostrand Reinhold, 1992), and Miller and Friedman, Photonic Rules of Thumb, 2004. ISBN 9780442012106
- ↑ James Byrnes. Unexploded Ordnance Detection and Mitigation. Springer 2009. pp. 21–22. ISBN 9781402092527.
- ↑ Hankwang (October 31, 2008). Terahertz radiation: Difference between revisions. pp. 1. http://en.wikipedia.org/w/index.php?title=Terahertz_radiation&diff=248835142&oldid=248831987. Retrieved 2012-08-05.
- ↑ Pozar, David M. (1993). Microwave Engineering Addison-Wesley Publishing Company. ISBN 0-201-50418-9.
- ↑ http://www.google.com/search?hl=en&defl=en&q=define:microwave&ei=e6CMSsWUI5OHmQee2si1DQ&sa=X&oi=glossary_definition&ct=title
- ↑ Rosemary Sullivant (May 31, 2011). Aquarius Studying Our Salty Seas From Space. Pasadena, California USA: Jet Propulsion Laboratory, NASA. http://www.nasa.gov/mission_pages/aquarius/news/aquarius20110525.html. Retrieved 2014-01-08.
External links
[edit | edit source]{{Charge ontology}}{{Physics resources}}
{{Radiation astronomy resources}}

