CORRECTED: with an aircraft wing, the lifting force does not come
from the difference in curvature between the top and bottom surfaces.
First read the entire: wings/lift webpage
Some books say that the lifting force appears
because the wing's upper surface is longer than the
lower surface.
They state that air dividing at the leading edge of the wing must rejoin
at the trailing edge, therefore the upper air stream must move faster, and
so the wing is pulled upwards by the Bernoulli Effect. This is not
correct: the air divided by the leading edge
does NOT rejoin at the
trailing edge, and there is no "race" to catch up.
The same books often
contain a misleading diagram showing a
flat-bottomed wing with flow lines of the surrounding air. (see below.)
This diagram actually shows a zero-lift condition. The lifting force is
zero because the air behind the airfoil does not descend. In order to
create
lift in a three-dimensional situation, a wing must deflect air
downwards.
Both the explanation and the diagram have serious problems. They wrongly
imply that inverted flight is impossible. They wrongly imply that an
aircraft with a symmetrical wing (a wing with equal
pathlengths above and below) will not fly. They also
wrongly suggest that an aircraft can violate the conservation of momentum
by remaining aloft without reacting against the air, and without causing a
downward motion of the air.
Yet upside-down flight is far from
impossible; it is a common aerobatic move. And many wings have equal
pathlengths, including even the thin cloth wings of the Wright Brothers'
flyer! And anyone standing under a slow, low-flying plane, or below the
thin, fast wings of a helicopter will know that there is a very great
downward flow of air below the wings. All of this indicates that there is
a serious problem with the "curved top, flat bottom" explanation. Below
is an alternative.
Go listen to NPR Science Friday Radio Archive, where physicist D. Anderson
debunks the various lifting-force myths.
Also see the NASA Aerodynamics Education site:
Here's my attempt at a correct explanation:
As a plane flies, the leading edges of its wings have little effect on
the
air, while the
trailing edges have a huge effect.
The wings' trailing edges always move through the air at an angle.
This "effective angle
of attack" causes the wing to apply a downward force to the air.
In order to create lift, the wing must be tilted. Or
rather than being tilted, the wings can be curved or "cambered",
which makes the trailing edge of the wing tilt downward at an angle.
The trailing edges of the wings cause the departing air to move downwards at an angle. As a
result, the wing is pushed
upwards and backwards. (These two pushes are called "lift" and "induced
drag.")
The tilted lower surface of the wing causes air to move down, but that's
not the only thing. The TOP of the wing also guides the
flowing air. This is called "flow attachment" or "Coanda Effect."
As the wing moves forward, the air ABOVE the wing moves down, and the
wing is forced upwards.
In other words, as any plane flies, its wings must send a stream of air
diagonally
downwards, and the wing acts like a 'reaction engine' just
like a jet engine or a rocket. Unless a wing is either tilted or
cambered, it cannot force the air downwards and cannot generate any
"lift."
It may help to imagine a hovering
helicopter: a helicopter can hover because its rotor applies a downward
force to the air, and the air applies an upward force to the rotor. As a
result, the air flows downwards while the upward force supports the
craft.
But like any airplane, a helicopter rotor is a moving wing, and it's this
small tilted wing which
sends the air downwards. Like any wing, helicopter rotors are reaction
engines, they
push air downwards, and the air pushes them upwards. They are not "sucked
upwards," and neither are airplanes.
You may have seen a plane's downwash of air in movies: a "cropduster"
plane sends out a trail of fertilizer mist, and the trail of mist does not
float, instead it moves immediately down into the crops, driven downward
by the moving air. Air from wings can even be dangerous: if a plane flies
too low, the downwash from its wings can knock people over.
The "Bernoulli effect" is still true. It explains how the top of the
wing is able to "pull downwards" on
the air flowing over it. And the Bernoulli Effect proves extremely useful
in calculations
of the lifting force during classes in airplane physics and during
experimental work in aerodynamics. But airplanes also obey Newton's
laws: accelerate some air downwards, and you'll experience an upwards
force.
Sound travels better through solids? No.
Many elementary textbooks say that sound travels better through solids and
liquids than through air, but they are incorrect. In fact, air, solids,
and liquids
are nearly transparent to sound waves. Some authors use an experiment to
convince us differently: place a solid ruler so it touches both a ticking
watch and your ear, and the sound becomes louder. Doesn't this prove that
wood is better than air at conducting sound? Not really, because sound
has an interesting property not usually mentioned in the books:
waves of sound traveling inside a solid will bounce off the air outside
the solid.
The experiment with the ruler merely proves that a wooden rod can act as a
sort of "tube," and it will guide sounds to your head which would
otherwise spread in all directions in the air. A hollow pipe can also be
used to guide the ticking sounds to your head, thus illustrating that air
is a good conductor after all. Sound in a solid has difficulty getting
past a crack in the solid, just as sound in the air has difficulty getting
past a wall. Solids, liquids, and air are nearly equal as sound
conductors.
It's true that the speed of sound differs in each material,
but this does not affect how well they conduct. "Faster" doesn't mean
"better." It is true that their transparency is not exactly the same, but
this only is important when sound travels a relatively great distance
through each material. It's also true that complex combinations of
materials conduct sound differently and may act as sound absorbers
(examples: water with clouds of bubbles, mixtures of various solids, air
filled with rain or snow.) And last: when you strike one object with
another, the
sound created inside the solid object is louder than the sound created in
the surrounding air. So, before we try to prove that solids are better
conductors, we had better make sure that we aren't accidentally putting
louder sound into the solids in the first place.
Gravity in space is zero? Wrong.
Everyone knows that the gravity in outer space is zero. Everyone is
wrong. Gravity in space is not zero, it can actually be fairly strong.
Suppose you climbed to the top of a ladder that's about 300 miles tall.
You would be up in the vacuum of space, but you would not be weightless at
all. You'd only weigh about fifteen percent less than you do on the
ground. While 300 miles out in space, a 115lb person would weigh about
100lb. Yet a spacecraft can orbit 'weightlessly' at the height of your
ladder! While you're up there, you might see the Space Shuttle zip right
by you. The people inside it would seem as weightless as always. Yet on
your tall ladder, you'd feel nearly normal weight. What's going on?
The reason that the shuttle astronauts act weightless is that
they're inside a container which is falling! If the shuttle were to
sit unmoving on top of your ladder (it's a strong ladder,) the shuttle
would no longer be falling, and its occupants would feel nearly normal
weight. And if you were to leap from your ladder, you would feel just as
weightless as an astronaut (at least you'd feel weightless until you hit
the ground!)
So, if the orbiting shuttle is really falling, why doesn't it hit
the earth? It's because the shuttle is not only falling down, it is
moving very fast sideways as it falls, so it falls in a curve. It moves
so fast that the curved path of its fall is the same as the curve of the
earth, so the Shuttle falls and falls and never comes down. Gravity
strongly affects the astronauts in a spacecraft: the Earth is strongly
pulling on them so they fall towards it. But they are moving sideways so
fast that they continually miss the Earth. This process is called
"orbiting," and the proper word for the seeming lack of gravity is called
"Free Fall." You shouldn't say that astronauts are "weightless," because
if you do, then anyone and anything that is falling would also be
"weightless." When you jump out of an airplane, do you become weightless?
And if you drop a book, does gravity stop affecting it; should you say it
becomes weightless? If so, then why does it fall? If "weight" is the
force which pulls objects towards the Earth, then this force is still
there even when objects fall.
So, to experience genuine free fall just like the
astronauts, simply jump into the air! Better yet, jump off a diving board
at the pool, or bounce on a trampoline, or go skydiving. Bungee-jumpers
know what the astronauts experience.
Space isn't remote at all. It's only an hour's drive away if your car
could go straight upwards. --Fred Hoyle
CORRECTED: For every action, there is not an
equal and opposite reaction.
Newton originally published his laws of motion in Latin, and in the
English translation, the word "action" was used in a different way than
it's usually used today. It was not used to suggest motion. Instead it
was used to mean "an acting upon." It was used in much the same way that
the word "force" is used today. What Newton's third law of motion means
is this:
For every "acting upon", there must be an equal
"acting upon" in the opposite direction.
Or in modern terms...
For every FORCE applied, there must be an equal FORCE
in the opposite direction.
So while
it's true that a skateboard does fly backwards when the rider steps off
it, these motions of "action" and "reaction" are not what
Newton
was investigating. Newton was actually referring to the fact that when
you push on something, it pushes back upon you equally, even if it
does not move. When a bowling ball pushes down on the Earth, the
Earth pushes up
on the bowling ball by the same amount. That is a good illustration of
Newton's third Law. Newton's Third Law can be
rewritten to say:
For every force there is an equal and opposite force.
Or "you cannot touch without being touched."
Or even
simpler: Forces always exist in pairs.
CORRECTED: Ben Franklin's kite was never struck by
lightning
Many people believe that Ben Franklin's kite was hit by a lightning
bolt, and this is how he proved that lightning is electrical. A number
of books and even some encyclopedias say the same thing. They are wrong.
When lightning strikes a kite, the electric current in the string is so
high that just the spreading electric currents in the ground can kill
anyone standing nearby, to say nothing of the person holding the string!
What Franklin actually did was to show that a kite would collect a tiny
bit of electrical charge-imbalance out of the sky during a thunderstorm.
Air is not a perfect insulator. The charges in a thunderstorm are
constantly leaking downwards through the air and into the ground.
Electric leakage through the air caused Franklin's kite and string to
become charged, and the hairs on the twine stood outwards. The twine was
then used to charge a metal key, and tiny sparks could then be drawn from
the key. Those tiny sparks were the only "lightning" in his experiment.
(He used a metal object because sparks cannot be directly drawn from
the twine; it's conductive, but not conductive enough to make sparks.)
His experiment told Franklin that some stormclouds carry strong electrical
charges, and it implied that lightning was just a large electric
spark.
The common belief that Franklin easily
survived a lightning strike is not just wrong, it is dangerous: it may
convince kids that it's OK to duplicate the kite experiment as long as
they "protect" themselves by holding a silk ribbon and employing a metal
key. Make no mistake, Franklin's experiment was extremely dangerous.
Lightning goes through miles of insulating air, and will not be stopped by
a piece of ribbon. If lightning had actually hit his kite, he would have
been gravely injured, and most
probably would have died instantly. See LIGHTNING SURVIVOR RESOURCES
The main lens of your eye is inside the eye? Not quite.
Some textbooks assume that the small lens found deep within the eyeball is the
eye's main lens, and the cornea of the eye is simply a protective window.
The textbook diagrams even depict light rays passing into the eye and only
bending as they pass through this internal lens. But in the human eye,
the small lens found within the eyeball is not the main imaging lens. The
cornea is actually the main lens; it is the strongly curved transparent
front surface of the eye. Most of the bending of the light occurs at the
place where the light enters the surface of the cornea. When you look at
your eye in the mirror, you are looking directly at the eye's main lens.
When you want to change the focusing power of your eye, you apply
"contact lenses" to the cornea surface, or you undergo surgery which
re-sculpts the cornea's curvature. The smaller lens inside the eye acts
only to alter the focus of the eye as a whole. Muscles change its shape
in order to correct the focus for near and far viewing. Without this
small internal lens, human vision would be blurry, and vision
would be
unable to accommodate for near and far views. But without the cornea lens,
[the human eye would be blind.] IMPROVED VERSION: without the
cornea
lens, human
vision would rely upon the pinhole-camera effect of the eye's pupil,
and vision would be incredibly blurry. Open your eyes underwater in
dimly-lit conditions to see what vision would be like without a cornea.
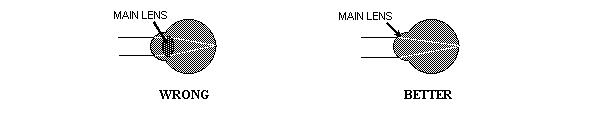
|
CORRECTED: when one prism splits light into colors, a
second identical prism cannot recombine them.
A single prism can split a sunbeam into a rainbow. Many children's
science books show how a second similar prism can be used to recombine the
colors. This is incorrect, two prisms do not work as shown. Prisms of two
different sizes can split and then focus the colors into momentary
recombination at a particular distance. With three prisms in a
special arrangement, the splitting and complete recombining of colors can
be accomplished. But books which depict one prism splitting the colors
and a second identical prism recombining the colors into a single white
beam are in error, and are no doubt the source of endless frustration for
those of us who try to duplicate the effect with real prisms.
The "rainbows" can also be recombined by placing a screen at just
the right place, and by bouncing the colors off many small mirrors so the
colored beams converge upon a screen. Recombination can also be done with
a convex lens or a concave mirror and a screen. I hope that very few
students will attempt to perform the color recombination experiment
depicted in their books, for disappointment awaits. (MORE)
Clouds, fog, and shower-room mist are water vapor? No.
All three things are made of small droplets of liquid water hanging in
the air. When water
evaporates,
it turns into a transparent gas called "water vapor." When it condenses
again, it can take the form of rain, snow, rivers, and oceans, but it also
can take the
form of clouds, mist, fog, etc. Fog can make surfaces wet, but not
because of condensation. Instead, the fog droplets collide with the
solid surface. Fog is liquid water, not a vapor. Fly an ultralight
aircraft
slowly through a large dense cloud, and you'll become damp. To look for
water vapor, look at the bubbles in rapidly boiling water. Look at the
small empty space at the spout of a boiling teakettle. Look at the far
end of the teakettle's plume of mist, where the mist seems to vanish into
the air. Look at the empty air above a wet surface. In these situations
you see nothing, and that's where the vapor is. Water vapor seems
invisible because it is transparent. Clouds and fog are not transparent.
They are composed of liquid droplets.
CORRECTED: raindrops don't have points!!
Nearly every
drawing of raindrops depicts them as having a sharp upper point. This is
wrong. Surface tension of water acts like a stretched "bag" around the
water, and unless some other force is acting, it pulls the water into a
spherical shape. Our eyes do see tiny droplets as a blur, but a flash
photograph reveals that small raindrops are nearly spherical. The larger
ones are distorted by the pressure of moving air, but this doesn't make
points, it makes them somewhat flattened. Think of it this way:
underwater bubbles are not pointed as they rise, just as falling water
drops are not pointed as they fall. And while it's true that the
symbol
for water is a droplet with a point, real water droplets look
nothing like
the symbol. And when water drips from a faucet, it never actually has a
point. Instead it has a narrow neck, and after the neck has snapped, it
is yanked back into the falling ball of water. See Dr. Fraser's BAD SCIENCE for
lots more about this.
Air is weightless? No.
We are not conscious of
air's weight because we are immersed within it. In the same way, even a
large bag of water seems weightless when it is immersed in a water tank.
The bag of water in the tank is
supported by buoyancy. In a similar way,
buoyancy from the atmosphere makes a bag of air seem weightless when it's
surrounded by air. One way to discover the real weight of air would be to
take a bag of air into a vacuum chamber. Another way is to weigh a
pressurized and an unpressurized football. A cubic meter of air at
sea-level pressure and
0C temperature has a mass of 1.2KG. The non-metric rule of thumb says
that the air that would fill a bathtub weighs about one pound.
Here's a simple way to
detect
the mass of air even though the air seems weightless: open an umbrella,
wiggle it slightly forwards and back, then close it and wiggle it again.
When you wiggle it when open, you can feel its increased mass because of
the air the umbrella must carry with it. (Ah, but then we must explain
the difference between weight and mass!)
CORRECTED: Filled and empty balloons do not demonstrate
the weight of air.
Many books contain an incorrect experiment which purports to directly
demonstrate that air has weight. A crude beam-balance is constructed
using a meter stick. Deflated rubber balloons are attached to the ends,
and the balance is adjusted. One balloon is then inflated, and that end
of the balance-beam is supposed to sag downwards. The demonstrator then
explains that a large amount of air weighs more than a small amount of
air.
Unfortunately this experiment isn't very honest. When immersed in
atmosphere, buoyancy
causes full and empty balloons to weigh the same. One balloon shouldn't
pull down the stick. But then why does the
above experiment work? Usually it doesn't! In fact, the experiment will
fail unless you know the trick: you must inflate the balloon near to
bursting. The experiment secretly relies on the fact that the air within
a high-pressure balloon is denser than air within a low pressure balloon.
Of course the demonstrator never mentions this to the students, and the
books which contain this demonstration don't mention density effects
either. Obviously the density effects do not directly demonstration
anything about the weight of air, so it's dishonest to tell students that
this demonstration can directly weigh some air.
To illustrate the problem, try this instead: attach two opened paper bags
to the balance, adjust it, then crush one bag so it contains little air.
The balance will not change. What does this teach your class; that
air is... weightless? Yet the air does have significant weight. We just
can't detect this weight directly by using paper bags. Or using
balloons.
Here's a way to make the experiment more honest. Perform the balance-beam
experiment again, but use two full balloons. Blow one balloon
really full so the rubber feels hard and the balloon is about to
pop. Blow up the second balloon so it is almost full, but still a
bit
stretchy. Try to keep the balloons almost the same size. Now the balance
will show that, even though the balloons look nearly the same, the "hard"
balloon is significantly heavier. Does this teach misleading things to
your class? Not really, instead it exposes the dishonesty of the original
demonstration. In truth, balloons filled with air will not weigh more
than empty balloons as long as they remain immersed in the atmosphere.
However, compressed air does weigh more than uncompressed
air. Perhaps this modified demonstration would be appropriate in more
advanced classes. But this website is about K-6 grade science.
Here's another way to think about it. Can we demonstrate the weight of
water to a fish?
What if we lived underwater, how could we use the balance-beam to measure
the weight of water directly? The answer is that we cannot. If a
water-filled balloon and a collapsed empty balloon were compared
underwater,
the experiment would show that they weigh the same, which seems to prove
that water is weightless. When underwater, a bag full of water weighs
just the same as a flattened bag which contains nothing. The situation
with air is similar: if we live our lives immersed within a sea of air,
we cannot use a balance to easily detect the actual weight of the air. (In
fact, a bathtub full of air weighs about a half kilogram, but we cannot
easily demonstrate this weight while living in an atmosphere.)
It's hard to teach the weight of water to the fishes, and hard to teach
the weight of air to human grade-schoolers. These misleading textbook
experiments could only work correctly if performed in a vacuum environment
(say, on the moon's surface.) We humans are like fish underwater: we're
not aware that our ocean of air has any weight. To demonstrate the
weight, we need to get out into a vacuum environment.
Or, to better demonstrate the weight of air directly, hook a heavy bottle
to a
vacuum pump, pump all the air out, seal it, then weigh the bottle. Break
the seal and let the air in, then weigh it again. The difference in
weight is the weight of the air contained in the bottle. Another: use a
balance to compare the weight of two vacuum-containing bottles, then open
one of them so it becomes filled with air. The bottles will then weigh
differently, and the difference is the true weight of the air in one
bottle. Or another: build a balance using upside-down paper bags, then
place a candle below one of them, then remove the candle again. That bag
rises, indicating that a volume of warm air weighs slightly less than a
volume of cool air. (Don't set the bag on fire!!) But note that this
candle experiment is a bit like the compressed balloon version, and it
says nothing simple and direct about the actual weight
of a volume of unheated air.
CORRECTED: in the everyday world, gases do not expand to
fill their containers.
What is the difference between a liquid and a gas? Both are "fluids",
both can flow. Gases are usually less dense than liquids, although
gases under fiercely high pressure can approach the density of liquids, so
that's not a good criterion. The main difference is that gases are a
different phase of matter: a gas can be made to condense into a liquid
form, and a liquid can be made to evaporate into gas. Another major
characteristic: because there are bonds between its particles, when a
liquid is placed into a vacuum environment, it will not immediately
and continuously expand, while a gas in a vacuum chamber will expand at
high velocity until it hits the container walls.
This is very different from the oft-quoted rule that "gases always expand
to fill their containers." This rule only works correctly if the
container is totally empty: the container must "contain" a good
vacuum beforehand. However, we all live in a gas-filled environment. All
our containers are pre-filled with air. In our environment, any new
quantity of gas will not expand, it will just sit there. It may slowly
diffuse outwards, but that's very different than the "expansion" being
discussed here. If
you squirt
some carbon dioxide out of a CO2 fire extinguisher, it will not instantly
expand to fill the room. Instead it will pour downwards like an invisible
fluid and form a pool on the
floor. It behaves similarly to dense sugar-water which was injected into
a tank of water: it pours downwards, and only after a very long time it
will mix with the rest of the water. "Mixing" is very different from
"expanding to fill!" The rule about gases does not involve mixing;
instead it involves compressibility and instant expansion into a vacuum.
In an air-filled room, dense gases act much like liquids; they can be
poured into a cup or bowl, poured out onto a tabletop, and then they
run off the edge onto the floor where they form an invisible mess. :)
Less dense gases will stay where they are put, like smoke or like food
coloring which has just been injected into a fishtank. Gas of even lesser
density rises and forms a pool on the ceiling. Only in the world
of the physicist, where "empty container" always implies a vacuum, does
the rule about gasses work properly.
CORRECTED: Shadows do vanish on cloudy days, but not
because the sun isn't bright enough.
Shadows appear when an object blocks a light source. The shape of the
shadow is created by the shape of the opaque object and by the
shape of the light source. On a cloudy day the whole sky acts as a light
source, and a person's shadow spreads out and becomes a dim fuzzy patch
which surrounds the person on the ground on all sides. The shadow is so
spread-out that it seems absent entirely. When the sun is visible, the
same shadow is concentrated in one specific place and becomes easy to see.
But even the shadows made by sunlight will have fuzzy borders, since the
sun is a small disk rather than a tiny dot. On cloudy days, the fuzzy
borders of your body's shadow become much much larger than the shadow
itself, so that the shadow seems to vanish.
CORRECTED: FRICTION IS NOT CAUSED BY SURFACE ROUGHNESS
Some books point to surface roughness as the explanation of sliding
friction. Surface roughness merely makes the moving surfaces bounce up
and down as they move, and any energy lost in pushing the surfaces apart
is regained when they fall together again. Friction is mostly caused by
chemical bonding between the moving surfaces; it is caused by stickiness.
Even scientists once believed this misconception, and they explained
friction as being caused by "interlocking asperites", the "asperites"
being microscopic bumps on surfaces. But the modern sciences of surfaces,
of abrasion, and of lubrication explain sliding friction in terms of
chemical bonding and "stick & slip" processes. The subject is still full
of unknowns, and new discoveries await those who make surface science
their profession
When thinking about friction, don't think about grains of sand on
sandpaper. Instead think about sticky adhesive tape being dragged along a
surface.
CORRECTED: NO, INFRARED LIGHT IS NOT A KIND OF HEAT
Infrared light is invisible light.
When any type of light is absorbed by an object, that object will be
heated. The infrared light from an electric heater feels hot for two
reasons: because surfaces seem black to IR light, and because the IR is
extremely bright light. Just because human eyes cannot see the
light which
causes the heating does not mean it's made of some mysterious entity
called "heat radiation." When bright light shines on an absorptive
surface, that surface heats up.
And this is no benign misconception. Those who fall under its sway may
also come to believe that *visible* light cannot heat surfaces (after all,
visible light is not "heat radiation?") Misguided science students may
wrongly believe that warm objects emit no microwaves (since only IR light
is "heat radiation"), even though hot objects actually do emit microwaves.
Or students may believe that the glow of red hot objects is somehow
different from the infrared glow of cooler objects. Or they may believe
that IR light is a form of "heat," and is therefore fundamentally
different from any other type of electromagnetic radiation.
In his book "
Clouds in a Glass of Beer," Physicist C. Bohren points out that this
"heat" misconception may have been started long ago, when early physicists
believed in the existence of three separate types of radiation: heat
radiation, light, and actinic radiation. Eventually they discovered that
all three were actually the same stuff: light. "Heat radiation" and
"actinic radiation" are simply invisible light of various frequencies.
Today we say "UV light" rather than "actinic radiation." Yet the obsolete
term "heat radiation" still lingers. Since human beings can only see
certain frequencies of light, it's easy to see how this sort of confusion
got started. Invisible light seems bizarre and mysterious when compared
to visible light. But "invisibility" is caused by the human eye, and is
not a property carried by the light. If humans could see all the light in
the infrared spectrum, we would say things like this: "of course
the
electric heater makes things hot at a distance, it is intensely
bright,
and bright light can heat up any surface which absorbs it."
PS, if
you're interested in physical science misconceptions,
Bohren's Book is an excellent resource. He's like me, and complains
about several specific misconceptions which keep his students from
understanding science.
CORRECTED: THERE ARE NOT SEVEN COLORS IN THE RAINBOW
Actually there's a very large number of distinct colors in any rainbow.
And neither are there sharp divisions between the bands of color, yet
numerous textbooks depict them. In reality, between yellow and green we
find yellow-green, and between green and yellowgreen is greenish
yellowgreen, and on and on. How many colors are in a rainbow? Thirty?
Sixty? It's not easy to say, for it depends on the particular eye, and
the particular rainbow. What of the teachers and students who look in
vain for the yellow-green in their textbook's depiction of rainbows?
They've crashed into a long-running textbook misconception: the strange
idea that rainbows have exactly seven distinct bands of color and no more,
and with nothing in between those uniform bands of 'official' color.
CORRECTED: ACTUALLY, THE EARTH'S NORTH AND SOUTH
MAGNETIC POLES RESIDE DEEP WITHIN THE EARTH'S CORE
Many textbooks have an erroneous diagram of the earth which shows
a bar magnet within it, and the ends of this bar magnet extend to just
beneath the earth's surface. These diagrams depict the magnet's field
lines as radiating from spots on the earth's surface. This is very
misleading. The earth's magnetic poles actually behave as if they're deep
within the earth, down inside the core. The Earth's magnetic field does
not come from a giant bar magnet, but if we imagine that it does,
then the imaginary "bar magnet" inside the earth is short, stubby,
disk-shaped, and part of the iron core deep inside the planet.
The typical textbook diagram is incorrect, and there are no intense
magnetic fields at the land surface near the earth's "north pole" and
"south pole." If you stand at the Earth's south magnetic pole, metals
aren't attracted to the ground more strongly than anywhere else. The
Geomagnetic "poles" on the earth's surface are not places where the field
is strong. They are simply the points on the landscape where the field
lines are perfectly vertical.
Proper diagrams
should instead show the field lines to be radiating from poles inside the
earth's core. They should show the field lines around the northern and
southern areas of the earth's surface as being approximately vertical and
parallel, not "radial" like a spiderweb and not concentrated into special
points on the surface.
Another error associated with the above: some books claim that the
earth's field at the magnetic poles is much stronger than elsewhere.
This is untrue. The field strength at the north magnetic pole above
Canada is about the same as the field strength in Virginia! And the
strongest field in the Earth's northern hemisphere does not appear at the
north magnetic pole at all, the North Pole actually has a weaker field
than elsewhere. The strongest fields in the northern hemisphere are not
in one but in two places: west of Hudson Bay in Canada, and in Siberia.
LINKS
LASER LIGHT IS "IN PHASE" LIGHT?
WRONG.
It's incorrect to say that "in laser light the waves are all in phase."
When two light waves traveling in the same direction combine, they
inextricably add together, they do not travel as two independent
"in-phase" waves. The photons in laser light are in phase, but the WAVES
are not. Instead, ideal laser light acts like a single, perfect wave.
When the light wave within a laser causes atoms to emit smaller, in
phase light waves, the result is not "in phase" light. Instead the result
is a single, more intense, amplified wave of light. In-phase emission
leads to amplification, not to multiple in-phase waves. If the atoms'
emissions weren't in phase, the result would not be light that's
out of phase. Instead the atoms would absorb light rather than amplifying
it.
Each atom in a laser contributes a tiny bit of light, but their light
vanishes into the main traveling wave. The light from each atom
strengthens the main beam, but loses its individuality in the process.
99 plus 1 equals 100, but if someone gives us 100, we cannot know if it is
made from 99 plus 1, or 98 plus 2, or 50 plus 50, etc.
Yes, it's true that all the photons associated with a single wave
of light are in phase. This might be one reason that people say that
laser light is "in phase" light. However, in-phase photons are nothing
unique, and they don't really explain coherence. Any EM sphere-wave or
plane-wave is made of in-phase photons. For example, all the photons
radiated from a radio broadcast antenna are also in phase, but we don't
say that these are special "in phase" radio waves, instead we just say
that they are waves with a spherical wavefront. Even if all the photons
in laser light are in phase, it is still incorrect to say "all the
WAVES are in phase." Photons are not waves. They are quanta, they
are particles, and they do not behave as small, individual "waves." Yes,
all the photons are in phase, but only because they are part of a single
plane-waves.
The light from a laser is basically a single, very powerful light wave.
Single waves are always in phase with themselves, but it's misleading to
imply that a single plane-wave or sphere-wave is something called an "in
phase" wave. Laser light could more accurately be called "pointsource"
light. Sphere waves or plane waves behave as if they were emitted from a
single tiny point. The physics term for this is "spatially coherent"
light. Light from light bulbs, flames, the sun, etc. are the opposite,
and are called "extended-source" light. Extended-source light comes from
a wide source, not from a point-source, and the waves coming from
different parts of the source will cross each other. Starlight and the
light from arc welders is "point-source" light and is quite similar to
laser light. Light from arc-welders and from distant stars has a higher
spatial coherence than light from most everyday light sources. (Note: the
sun is a star, correctly implying that light becomes more and more
spatially coherent as it moves far from its source. This is a clue as to
the real reason that lasers give spatially coherent light! (See
below)
CORRECTED: LASER LIGHT IS NOT PARALLEL LIGHT
Light from most lasers is not parallel light. However, if laser light is
passed through the correct lenses, it can be formed into a tight, parallel
beam. The same is not true for light from an ordinary light bulb. If
light from a light bulb were passed through the same lenses, it would form
a spreading beam, and an image of the lightbulb would be projected into
the distance. Laser light can form beams because a laser is a pointsource,
and when you project the image of a pointsource into the distance, you
form a narrow parallel beam! However, it is simply wrong to state that
laser light is inherently parallel light. Laser light can be
formed into parallel light, while the light from ordinary sources cannot.
Most types of lasers actually emit spreading, non-parallel light. Lasers
in CD players and in "laser pointers" are semiconductor diode lasers.
They create cone-shaped light beams, and if a parallel beam is desired,
they require a focusing lens. The same is true for the lasers in
inexpensive "laser pointers." Take apart an old laser-pointer, and you'll
find the plastic lens in front of the diode laser inside.
Classroom "HeNe" lasers also create spreading light. The laser tube
within a typical classroom laser contains at least one curved mirror
(called a "confocal" arrangement,) and it creates light in the form of a
spreading cone. It's a little-known fact that manufacturers of classroom
lasers traditionally place a convex lens on the end of their laser tubes
in order to shape the spreading light into a parallel beam. While it's
true that a narrow beam is convenient, I suspect that part of their reason
is to force the laser to fit our stereotype that all lasers produce thin,
narrow light beams. The manufacturers could save money by selling "real"
lensless laser tubes having spreading beams. But customers would
complain, wouldn't they? We have been brought up to believe that laser
light is parallel light.
CORRECTED: LASERS EMIT COHERENT LIGHT, BUT NOT
BECAUSE THE
ATOMS EMIT IN-PHASE LIGHT WAVES
In-phase emission causes the amplification of light, it doesn't
cause coherent light. Because the atoms emit light in phase with incoming
light, they will amplify the light, but they amplify incoherent light too,
and they don't make it coherent. The coherence of laser light has another
source...
Laser light has two main characteristics: it is "monochromatic" or very
pure in frequency (this also is called "temporally coherent.") Laser
light also has a point-source character of sphere waves and plane waves
(also called "spatially coherent.")
Even fairly advanced textbooks fail to give the real reason why laser
light is spatially coherent. They usually point out that the laser's
atoms all emit their light in phase, and pretend that this leads to
spatial coherence. Wrong. It is true that the fluorescing atoms in a
laser all emit light that's in-phase with the waves already traveling
between the mirrors. But the in-phase emission only creates
amplification of the traveling waves, it does not create spatially
coherent light. For example, if you were to feed incoherent light
into a HeNe laser tube, the atoms would emit in-phase waves, and the laser
would amplify the light. But the brighter light would still be
incoherent! Lasers certainly can amplify the coherent wave which
is trapped between their mirrors. But how did the light within the laser
get to be coherent in the first place?
Lasers create coherent light because of their mirrors.
The mirrors in a laser form a resonant cavity which preserves coherent
light while rejecting incoherent light. How does it work? Imagine a
simplified laser having flat, parallel mirrors. As light bounces between
the mirrors, the light "thinks" that it's traveling down an infinitely
long virtual tunnel. (Have you ever held up two mirrors facing each
other? Then you've seen this infinite tunnel.) When a laser is first
turned on, it fluoresces; it emits light which is not coherent.
Different random light waves start out from different parts of the laser.
After a few thousand mirror bounces, all the waves have added and
subtracted to form just one single EM wave. In the case of flat-mirror
lasers, this wave is a nearly perfect plane wave. A single plane wave is
coherent (to be incoherent, you must have at least two different
waves.)
This can be a bit confusing. After all, the individual atoms each emit a
wave. Don't all these waves add up to messy incoherent light? No. The
in-phase emission preserves the existing coherence as it amplifies. It's
true that each atom emits light waves in all directions. However, these
sideways waves from all the atoms will cancel each other out, and only the
waves that travel in the same direction as the incoming light will be
preserved. It's as if the atoms "know" which direction to send out their
beam. But in reality, the atoms don't need to know the beam direction.
Instead, they just emit a light wave which is in phase with the incoming
light, and for this reason the wave from the atom will cancel out
everywhere except in a line with the incoming light. If the light in a
laser were already coherent, then the atoms will amplify it but
won't make it more coherent. The coherence comes from the great distance
that the light has traveled as it bounced between the mirrors.
A similar thing happens with starlight: starlight is coherent! Starlight
travels far from its original source and all the waves from different
parts of the star will add up to form a wave with a single planar
wavefront. Light from distant stars is spatially coherent, even though
sunlight is not, yet the sun is a star too. The farther the light travels
from its source, the more it approaches the shape of a perfect plane wave.
And a perfect plane wave is perfectly coherent. Laser light is spatially
coherent because, among other things, the bouncing light has traveled
millions of miles between mirrors, and all the various competing waves
have melded together to form a single pure plane-wave or
sphere-wave.
P.S. The pure color (monochrome) laser light is also
created by the
mirrors. Huh? Yes, but the reason for this is not totally
straightforward (and it's quite a bit beyond the K-6 level of these
webpages!)
The two mirrors of a laser can trap a standing wave of light. The space
between the mirrors is like the string of a guitar: there can be a
fundamental wave, or overtone waves, or complicated waves which are a
mixture of these. But waves of non-overtone frequencies cannot exist
between the mirrors. Since the distance between the crests of a light
wave is very small, lots of different overtones can fit between the
mirrors, and each overtone is a slightly-different pure color of light.
Light from a neon sign is reddish, but it doesn't have the extreme purity
of laser light. Now for the weird part: when a Helium-Neon laser first
operates, many different overtones of red light are amplified and the beam
contains many slightly-different colors of red at the same time. It's not
yet monochromatic. As time goes on, some of these colors are amplified a
bit more than others, and this uses up the available energy coming from
the laser power supply. In other words, the different waves start
competing for limited resources! Just one wave "wins" in the end, and all
of the other overtones drop out of the running. The laser light is not
just red light. Instead it is a single pure overtone-wave, a pure
frequency where the string of waves just perfectly fits in the space
between the two mirrors. Change the spacing of the laser's mirrors (for
example by heating a glass HeNe laser tube,) and you change the frequency
of the light.
CORRECTED: IRON AND STEEL ARE NOT THE ONLY STRONGLY
MAGNETIC MATERIALS
There are numerous others. Nickel and
Cobalt
metals are very magnetic. (U.S. "nickel" coins contain copper which
spoils the effect, so try Canadian nickels made before 1985.) Most other
materials are "diamagnetic," and are repelled visibly by very strong
magnets, although some materials are "paramagnetic" and are attracted.
Supercold liquid oxygen is attracted by magnets. Some but not all types
of stainless steel are nonmagnetic. There are even some metals which are
individually nonmagnetic, but which become strongly magnetic when mixed
together, chromium and platinum for example, and compounds of manganese
and bismuth.
CORRECTED: RE-ENTERING SPACE CAPSULES ARE
NOT
HEATED BY AIR
FRICTION
They are heated as they plow into the atmosphere and
compress the air ahead of them. Ever pump up a bicycle tire and discover
that the pump and the tire have become hot? The same effect causes
spacecraft and supersonic aircraft to heat up as they compress the air at
their leading edges. The heat doesn't come from *rubbing* upon the air,
it comes from *squeezing* the air. This applies mostly to blunt objects
such as Apollo reentry vehicles. It does not apply as much to the Space
Shuttle: with wings oriented mostly edge-on to the moving air, the
surfaces of the Shuttle are heated by friction. But when the
Shuttle
first reenters the atmosphere, the bottom of the craft faces forwards,
and in that case the Shuttle is heated by air compression, not by
friction.
CORRECTED: CARS AND AIRPLANES ARE NOT SLOWED DOWN BY
AIR FRICTION
They are slowed because it takes energy to stir the air. While
direct
friction between the air and the car's surface does play a part, the work
done in stirring the air far exceeds the work done in direct frictional
heating. If vehicles did not send air swirls and vortices spinning off as
they moved, they would barely be slowed by the air at all. Eventually the
swirling air is slowed by friction and ends up warmer, but this occurs
long after the vehicle has passed.
CORRECTED: THE NORTH MAGNETIC POLE OF THE EARTH IS
NOT IN THE NORTH
Opposite poles attract. If we hold two bar magnets near each
other,
the "N" pole of one magnet is attracted by the "S" pole of another. If we
suspend a bar magnet by a thread, the "N" pole of that magnet will
point... toward the Earth's north!
Something is wrong here.
Shouldn't the
"N" pole of a magnet point towards the "S" of the Earth? Alike poles
should repel, not attract. Either the "N" and "S" printed on all bar
magnets is
reversed, or the "N" and "S" on the Earth is backwards. Which is
it?
This problem has a simple solution. Physicists define "N-type" magnetic
poles as being the north-pointing ends of compasses and magnets. This
definition is built into all of modern science and engineering and is part
of Maxwell's equations. Wind an electromagnet coil, see which end points
towards the Earth's North Pole, and that end is the "N pole" of the
electromagnet. And this means that the magnetic pole found deep inside
the northern hemisphere of the Earth is a south-type magnetic pole. The
Earth's northern magnetic pole is an S! It has to be this way, otherwise
it would not attract the N-pole of a compass.
This is a long-standing but arbitrary physical standard, much the same as
defining electrons as being negative. Like it or not, we are stuck with
negative electrons, with seconds which last about 1/100,000 of a day, with
backwards Earth poles, with centimeters which are about as wide as a small
finger, etc.
Interesting email
msgs on magnetic polarity
See Dexter Magnetics for more on this.
Also try this Google
search
CORRECTED: ACTUALLY THERE ARE NO SODIUM CHLORIDE MOLECULES IN SALT WATER
Salt is not made of NaCl molecules. Salt is made of a three-dimensional
checkerboard of oppositely charged atoms of sodium and chlorine. A salt
crystal is like a single gigantic molecule of ClNaClNaClNaClNaClNaClNa.
When salt dissolves, it turns into independent atoms. Salt water is not
full of "sodium chloride." Instead it is full of sodium and chlorine!
The atoms are not poisonous and reactive like sodium metal and chlorine
gas because they are electrically charged atoms called "ions." The sodium
atoms are missing their outer electron. Because of this, the remaining
electrons behave as a filled electron shell, so they cannot easily react
and form chemical bonds with other atoms except by electrical attraction.
The chlorine has one extra electron and its outer electron shell is
complete, so like sodium it too cannot bond with other atoms. These
oppositely charged
atoms can attract each other and form a salt crystal, but when that
crystal dissolves in water, the electrified atoms are pulled away from
each other as the water molecules surround them, and they float through
the water separately.
CORRECTED: LIGHT AND RADIO WAVES DO NOT ALWAYS TRAVEL AT "THE
SPEED OF LIGHT"
They only travel at the "speed of light" (186,000 miles per second) while
moving through a perfect vacuum. Light waves travel a bit slower in the
air, and they travel lots slower when moving through glass. Why
does
light bend when it enters glass at an angle? Because the waves SLOW DOWN.
Why can a prism split white light into a spectrum? Because within the
glass the different wavelengths of light waves have different
speeds
And while the numerical value for the speed of light in a vacuum, "c," is
very important in all facets of physics, as far as light waves are
concerned there is no single unique speed called "The Speed Of Light."
[note for advanced students: ok ok, I'll add this: light *waves* within a
transparent medium are slow, even though the wave's photons are thought to
jump from atom to atom always at a speed of c. But such ideas are not
very honest, since whenever we only pay attention to the vacuum between
particles in a solid, we stop treating the solid as a "uniform transparent
medium." ]