| Entry |
|
|---|
| Name |
Clopidogrel bisulfate (USP);
Clopidogrel sulfate (JP18);
Clopidogrel sulfate tablets (JP18);
Clopidogrel hydrogen sulphate;
Isocover (TN);
Plavix (TN) |
|---|
| Product |
|
|---|
| Generic |
CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (A-S Medication Solutions), CLOPIDOGREL (American Health Packaging), CLOPIDOGREL (Amneal Pharmaceuticals LLC), CLOPIDOGREL (Aphena Pharma Solutions - Tennessee), CLOPIDOGREL (Apotex Corp.), CLOPIDOGREL (Ascend Laboratories), CLOPIDOGREL (Aurobindo Pharma Limited), CLOPIDOGREL (AvPAK), CLOPIDOGREL (Bryant Ranch Prepack), CLOPIDOGREL (Bryant Ranch Prepack), CLOPIDOGREL (Camber Pharmaceuticals), CLOPIDOGREL (DIRECT RX), CLOPIDOGREL (Golden State Medical Supply), CLOPIDOGREL (Legacy Pharmaceutical Packaging), CLOPIDOGREL (Macleods Pharmaceuticals Limited), CLOPIDOGREL (Major Pharmaceuticals), CLOPIDOGREL (NCS HealthCare of KY), CLOPIDOGREL (NorthStar RxLLC), CLOPIDOGREL (Novadoz Pharmaceuticals LLC), CLOPIDOGREL (NuCare Pharmaceuticals), CLOPIDOGREL (NuCare Pharmaceuticals), CLOPIDOGREL (POLYGEN PHARMACEUTICALS), CLOPIDOGREL (Proficient Rx LP), CLOPIDOGREL (Proficient Rx LP), CLOPIDOGREL (Proficient Rx LP), CLOPIDOGREL (REMEDYREPACK), CLOPIDOGREL (REMEDYREPACK), CLOPIDOGREL (REMEDYREPACK), CLOPIDOGREL (RPK Pharmaceuticals), CLOPIDOGREL (RedPharm Drug), CLOPIDOGREL (Redpharm Drug), CLOPIDOGREL (Teva Pharmaceuticals USA), CLOPIDOGREL (Vensun Pharmaceuticals), CLOPIDOGREL (Viona Pharmaceuticals), CLOPIDOGREL (Westminster Pharmaceuticals), CLOPIDOGREL (Zydus Lifesciences Limited), CLOPIDOGREL 75MG (Coupler LLC), CLOPIDOGREL BISULFATE (ACI Healthcare USA), CLOPIDOGREL BISULFATE (Bryant Ranch Prepack), CLOPIDOGREL BISULFATE (Bryant Ranch Prepack), CLOPIDOGREL BISULFATE (Cardinal Health 107), CLOPIDOGREL BISULFATE (Dr.Reddy's Laboratories Limited), CLOPIDOGREL BISULFATE (Dr.Reddy's Laboratories Limited), CLOPIDOGREL BISULFATE (Graviti Pharmaceuticals Private Limited), CLOPIDOGREL BISULFATE (Major Pharmaceuticals), CLOPIDOGREL BISULFATE (McKesson Corporation dba SKY Packaging), CLOPIDOGREL BISULFATE (NuCare Pharmaceuticals), CLOPIDOGREL BISULFATE (PD-Rx Pharmaceuticals), CLOPIDOGREL BISULFATE (Preferred Pharmaceuticals), CLOPIDOGREL BISULFATE (Proficient Rx LP), CLOPIDOGREL BISULFATE (Quallent Pharmaceuticals Health LLC), CLOPIDOGREL BISULFATE (RADHA PHARMACEUTICALS), CLOPIDOGREL BISULFATE (REMEDYREPACK), CLOPIDOGREL BISULFATE (REMEDYREPACK), CLOPIDOGREL BISULFATE (RedPharm Drug), CLOPIDOGREL BISULFATE (ScieGen Pharmaceuticals), CLOPIDOGREL BISULFATE (Sun Pharmaceutical Industries), CLOPIDOGREL BISULFATE (Torrent Pharmaceuticals Limited) |
|---|
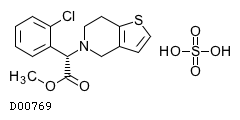
| Formula |
C16H16ClNO2S. H2SO4
|
|---|
| Exact mass |
419.0264
|
|---|
| Mol weight |
419.90
|
|---|
| Structure |
|
|---|
| Simcomp |
|
|---|
| Class |
|
|---|
| Remark |
| Therapeutic category: | 3399 |
| Product (DG00155): | D00769<JP/US> |
| Product (mixture): | D10513<JP> |
|
|---|
| Efficacy |
Anticoagulant, Platelet aggregation inhibitor, Purinergic receptor P2Y12 antagonist |
|---|
| Disease |
Myocardial infarction [DS: H01730] |
|---|
| Comment |
Thienopyridine derivative
CYP2C19 poor metabolizer status is associated with diminished response to Clopidogrel.
|
|---|
| Target |
|
|---|
| Pathway |
|
|---|
| Metabolism |
Enzyme: CYP2C19 [HSA: 1557]; CYP3A4 [HSA: 1576], CYP1A2 [HSA: 1544], CYP2B6 [HSA: 1555]
|
|---|
| Interaction |
CYP inhibition: CYP2C8 [HSA: 1558]
|
|---|
| Structure map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
B BLOOD AND BLOOD FORMING ORGANS
B01 ANTITHROMBOTIC AGENTS
B01A ANTITHROMBOTIC AGENTS
B01AC Platelet aggregation inhibitors excl. heparin
B01AC04 Clopidogrel
D00769 Clopidogrel bisulfate (USP) <JP/US>
USP drug classification [BR:br08302]
Blood Products and Modifiers
Platelet Modifying Agents
Adenosine Diphosphate P2Y12 Inhibitors
Clopidogrel
D00769 Clopidogrel bisulfate (USP)
Therapeutic category of drugs in Japan [BR:br08301]
3 Agents affecting metabolism
33 Blood and body fluid agents
339 Miscellaneous
3399 Others
D00769 Clopidogrel bisulfate (USP); Clopidogrel sulfate (JP18); Clopidogrel sulfate tablets (JP18)
Drug groups [BR:br08330]
Blood modifier agent
DG01950 Antithrombotic agent
DG01712 Antiplatelet agent
DG01808 Thienopyridine
DG00155 Clopidogrel
D00769 Clopidogrel bisulfate
DG01809 P2Y12 receptor antagonist
DG01808 Thienopyridine
DG00155 Clopidogrel
D00769 Clopidogrel bisulfate
Metabolizing enzyme substrate
DG01892 CYP1A2 substrate
DG00155 Clopidogrel
D00769 Clopidogrel bisulfate
DG02919 CYP2B6 substrate
DG00155 Clopidogrel
D00769 Clopidogrel bisulfate
DG01639 CYP2C19 substrate
DG00155 Clopidogrel
D00769 Clopidogrel bisulfate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00155 Clopidogrel
D00769 Clopidogrel bisulfate
Metabolizing enzyme inhibitor
DG01641 CYP2C8 inhibitor
DG00155 Clopidogrel
D00769 Clopidogrel bisulfate
Target-based classification of drugs [BR:br08310]
G Protein-coupled receptors
Rhodopsin family
Purine / pyrimidine
P2RY12
D00769 Clopidogrel bisulfate (USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00769 Clopidogrel sulfate
D00769 Clopidogrel sulfate tablets
New drug approvals in Europe [br08329.html]
European public assessment reports (EPAR) authorised medicine
D00769
New drug approvals in Japan [br08318.html]
Drugs with new active ingredients
D00769
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00769
Pharmacogenomic biomarkers [br08341.html]
Polymorphisms and mutations affecting drug response
D00769
Drug groups [BR:br08330]
Blood modifier agent
DG01950 Antithrombotic agent
DG01712 Antiplatelet agent
DG01808 Thienopyridine
DG00155 Clopidogrel
DG01809 P2Y12 receptor antagonist
DG01808 Thienopyridine
DG00155 Clopidogrel
Metabolizing enzyme substrate
DG01892 CYP1A2 substrate
DG00155 Clopidogrel
DG02919 CYP2B6 substrate
DG00155 Clopidogrel
DG01639 CYP2C19 substrate
DG00155 Clopidogrel
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00155 Clopidogrel
Metabolizing enzyme inhibitor
DG01641 CYP2C8 inhibitor
DG00155 Clopidogrel
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 26
1 N1y N 21.9223 -14.0768
2 C1c C 20.7116 -14.7786
3 C1x C 23.1271 -14.7786
4 C1x C 21.9164 -12.6791
5 C8y C 19.5011 -14.0768
6 C7a C 20.7116 -16.1766
7 C8y C 24.3378 -14.0827
8 C1x C 23.1271 -11.9773
9 C8y C 19.5011 -12.6791
10 C8x C 18.2904 -14.7786
11 O7a O 19.5011 -16.8782
12 O6a O 21.9223 -16.8725
13 C8y C 24.3378 -12.6791
14 C8x C 25.6771 -14.5154
15 C8x C 18.2904 -11.9773
16 X Cl 20.7116 -11.9773
17 C8x C 17.0797 -14.0768
18 C1a C 18.2904 -16.1766
19 S2x S 25.6712 -12.2405
20 C8x C 26.5018 -13.3751
21 C8x C 17.0797 -12.6791
22 S4a S 29.6520 -14.3900
23 O1d O 29.6520 -12.9900
24 O1d O 29.6520 -15.7900
25 O1d O 28.2519 -14.3900
26 O1d O 31.0519 -14.3900
BOND 27
1 2 5 1
2 2 6 1 #Down
3 3 7 1
4 4 8 1
5 5 9 1
6 5 10 2
7 6 11 1
8 6 12 2
9 7 13 2
10 7 14 1
11 9 15 2
12 9 16 1
13 10 17 1
14 11 18 1
15 13 19 1
16 14 20 2
17 15 21 1
18 8 13 1
19 17 21 2
20 19 20 1
21 1 2 1
22 1 3 1
23 1 4 1
24 22 23 2
25 22 24 2
26 22 25 1
27 22 26 1
|
|---|
|